Nutr Res Pract.
2023 Dec;17(6):1238-1254. 10.4162/nrp.2023.17.6.1238.
The effects of dietary self-monitoring intervention on anthropometric and metabolic changes via a mobile application or paper-based diary: a randomized trial
- Affiliations
-
- 1Division of Cancer Prevention, National Cancer Control Institute, National Cancer Center, Goyang 10408, Korea
- 2School of Bio-Medical Science, Korea University, Sejong 30019, Korea
- 3Department of Food and Nutrition, College of Human Ecology, Seoul National University, Seoul 08826, Korea
- 4Department of Food and Nutrition, Sookmyung Women’s University, Seoul 04310, Korea
- 5School of Global Sport Studies, Korea University, Sejong 30019, Korea
- 6College of Pharmacy, Korea University, Sejong 30019, Korea
- 7The Research Institute of Human Ecology, Seoul National University, Seoul 08826, Korea
- KMID: 2548889
- DOI: http://doi.org/10.4162/nrp.2023.17.6.1238
Abstract
- BACKGROUND/OBJECTIVES
Weight loss via a mobile application (App) or a paper-based diary (Paper) may confer favorable metabolic and anthropometric changes.
SUBJECTS/METHODS
A randomized parallel trial was conducted among 57 adults whose body mass indices (BMIs) were 25 kg/m 2 or greater. Participants randomly assigned to either the App group (n = 30) or the Paper group (n = 27) were advised to record their foods and supplements through App or Paper during the 12-week intervention period. Relative changes of anthropometries and biomarker levels were compared between the 2 intervention groups. Untargeted metabolic profiling was identified to discriminate metabolic profiles.
RESULTS
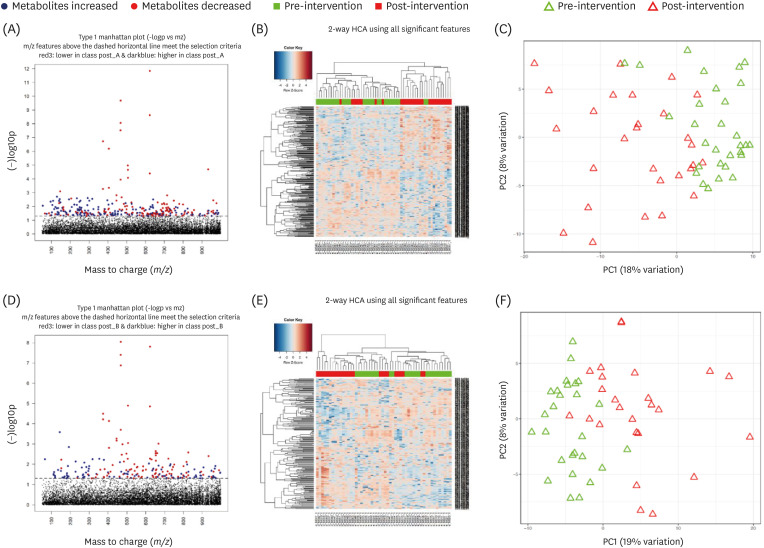
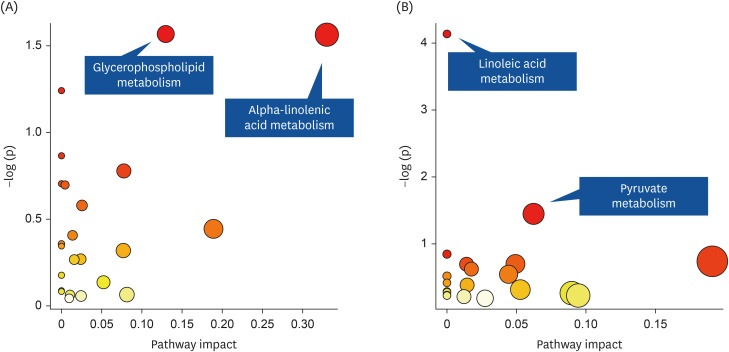
Out of the 57 participants, 54 participants completed the trial. Changes in body weight and BMI were not significantly different between the 2 groups (P = 0.11). However, body fat and low-density lipoprotein (LDL)-cholesterol levels increased in the App group but decreased in the Paper group, and the difference was statistically significant (P = 0.03 for body fat and 0.02 for LDL-cholesterol). In the metabolomics analysis, decreases in methylglyoxal and (S)-malate in pyruvate metabolism and phosphatidylcholine (lecithin) in linoleic acid metabolism from pre- to post-intervention were observed in the Paper group.
CONCLUSIONS
In the 12-week randomized parallel trial of weight loss through a App or a Paper, we found no significant difference in change in BMI or weight between the App and Paper groups, but improvement in body fatness and LDL-cholesterol levels only in the Paper group under the circumstances with minimal contact by dietitians or health care providers. Trial Registration: Clinical Research Information Service Identifier: KCT0004226
Figure
Reference
-
1. World Health Organization. Obesity and overweight [Internet]. Geneva: World Health Organization;2021. cited 2016 April 24. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.2. Korea Centers for Disease Control and Prevention. Korea Health Statistics 1998: Korea National Health and Nutrition Examination Survey (KNHANES I). Cheongju: Korea Centers for Disease Control and Prevention;2012.3. Korea Centers for Disease Control and Prevention. Korea Health Statistics 2021: Korea National Health and Nutrition Examination Survey (KNHANES VIII-3). Cheongju: Korea Centers for Disease Control and Prevention;2022.4. World Health Organization. Atlas of eHealth country profiles 2015: the use of eHealth in support of universal health coverage based on the findings of the 2015 global survey on eHealth [Internet]. Geneva: World Health Organization;2020. cited 2015 April 23. Available from: https://www.who.int/publications/i/item/9789241565219.5. Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, Patel V, Haines A. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013; 10:e1001362. PMID: 23349621.6. Fakih El Khoury C, Karavetian M, Halfens RJ, Crutzen R, Khoja L, Schols JM. The effects of dietary mobile apps on nutritional outcomes in adults with chronic diseases: a systematic review and meta-analysis. J Acad Nutr Diet. 2019; 119:626–651. PMID: 30686742.7. Turner-McGrievy GM, Wilcox S, Boutté A, Hutto BE, Singletary C, Muth ER, Hoover AW. The dietary intervention to enhance tracking with mobile devices (DIET Mobile) study: a 6-month randomized weight loss trial. Obesity (Silver Spring). 2017; 25:1336–1342. PMID: 28600833.8. Stephens JD, Yager AM, Allen J. Smartphone technology and text messaging for weight loss in young adults: a randomized controlled trial. J Cardiovasc Nurs. 2017; 32:39–46. PMID: 26646593.9. Ross KM, Wing RR. Impact of newer self-monitoring technology and brief phone-based intervention on weight loss: a randomized pilot study. Obesity (Silver Spring). 2016; 24:1653–1659. PMID: 27367614.10. Thomas JG, Bond DS, Raynor HA, Papandonatos GD, Wing RR. Comparison of smartphone-based behavioral obesity treatment with gold standard group treatment and control: a randomized trial. Obesity (Silver Spring). 2019; 27:572–580. PMID: 30779333.11. Wang J, Cai C, Padhye N, Orlander P, Zare M. A behavioral lifestyle intervention enhanced with multiple-behavior self-monitoring using mobile and connected tools for underserved individuals with type 2 diabetes and comorbid overweight or obesity: pilot comparative effectiveness trial. JMIR Mhealth Uhealth. 2018; 6:e92. PMID: 29636320.12. Wharton CM, Johnston CS, Cunningham BK, Sterner D. Dietary self-monitoring, but not dietary quality, improves with use of smartphone app technology in an 8-week weight loss trial. J Nutr Educ Behav. 2014; 46:440–444. PMID: 25220777.13. Spring B, Pellegrini CA, Pfammatter A, Duncan JM, Pictor A, McFadden HG, Siddique J, Hedeker D. Effects of an abbreviated obesity intervention supported by mobile technology: the ENGAGED randomized clinical trial. Obesity (Silver Spring). 2017; 25:1191–1198. PMID: 28494136.14. Dunn WB, Ellis DI. Metabolomics: current analytical platforms and methodologies. Trends Analyt Chem. 2005; 24:285–294.15. Dunn WB, Erban A, Weber RJ, Creek DJ, Brown M, Breitling R, Hankemeier T, Goodacre R, Neumann S, Kopka J, et al. Mass appeal: metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics. 2013; 9:44–66.16. Geidenstam N, Al-Majdoub M, Ekman M, Spégel P, Ridderstråle M. Metabolite profiling of obese individuals before and after a one year weight loss program. Int J Obes. 2017; 41:1369–1378.17. Kang M, Yoo HJ, Kim M, Kim M, Lee JH. Metabolomics identifies increases in the acylcarnitine profiles in the plasma of overweight subjects in response to mild weight loss: a randomized, controlled design study. Lipids Health Dis. 2018; 17:237. PMID: 30322392.18. Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, D.C.: The National Academies Press;2005.19. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37(Suppl 1):S81–S90. PMID: 24357215.20. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106:3143–3421. PMID: 12485966.21. Uppal K, Ma C, Go YM, Jones DP, Wren J. xMWAS: a data-driven integration and differential network analysis tool. Bioinformatics. 2018; 34:701–702. PMID: 29069296.22. Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, Cheng D, Jewell K, Arndt D, Sawhney S, et al. HMDB: the Human Metabolome Database. Nucleic Acids Res. 2007; 35:D521–D526. PMID: 17202168.23. Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009; 37:W652–W660. PMID: 19429898.24. Xia J, Wishart DS. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics. 2010; 26:2342–2344. PMID: 20628077.25. Ahn JS, Lee H, Kim J, Park H, Kim DW, Lee JE. Use of a smartphone app for weight loss versus a paper-based dietary diary in overweight adults: randomized controlled trial. JMIR Mhealth Uhealth. 2020; 8:e14013. PMID: 32735225.26. Burke LE, Styn MA, Sereika SM, Conroy MB, Ye L, Glanz K, Sevick MA, Ewing LJ. Using mHealth technology to enhance self-monitoring for weight loss: a randomized trial. Am J Prev Med. 2012; 43:20–26. PMID: 22704741.27. Laing BY, Mangione CM, Tseng CH, Leng M, Vaisberg E, Mahida M, Bholat M, Glazier E, Morisky DE, Bell DS. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients: a randomized, controlled trial. Ann Intern Med. 2014; 161:S5–12. PMID: 25402403.28. Wiss DA, Avena N, Rada P. Sugar addiction: from evolution to revolution. Front Psychiatry. 2018; 9:545. PMID: 30464748.29. Avena NM, Bocarsly ME. Dysregulation of brain reward systems in eating disorders: neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharmacology. 2012; 63:87–96. PMID: 22138162.30. Pahlavani M, Razafimanjato F, Ramalingam L, Kalupahana NS, Moussa H, Scoggin S, Moustaid-Moussa N. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes. J Nutr Biochem. 2017; 39:101–109. PMID: 27833050.31. Serini S, Fasano E, Piccioni E, Cittadini AR, Calviello G. Dietary n-3 polyunsaturated fatty acids and the paradox of their health benefits and potential harmful effects. Chem Res Toxicol. 2011; 24:2093–2105. PMID: 21902224.32. Richard JP. Mechanism for the formation of methylglyoxal from triosephosphates. Biochem Soc Trans. 1993; 21:549–553. PMID: 8359530.33. Lu M, Zhou L, Stanley WC, Cabrera ME, Saidel GM, Yu X. Role of the malate-aspartate shuttle on the metabolic response to myocardial ischemia. J Theor Biol. 2008; 254:466–475. PMID: 18603266.34. Hirata F, Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980; 209:1082–1090. PMID: 6157192.35. DeLong CJ, Shen YJ, Thomas MJ, Cui Z. Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J Biol Chem. 1999; 274:29683–29688. PMID: 10514439.36. Jacobs RL, Zhao Y, Koonen DP, Sletten T, Su B, Lingrell S, Cao G, Peake DA, Kuo MS, Proctor SD, et al. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J Biol Chem. 2010; 285:22403–22413. PMID: 20452975.37. Schippers M, Adam PC, Smolenski DJ, Wong HT, de Wit JB. A meta-analysis of overall effects of weight loss interventions delivered via mobile phones and effect size differences according to delivery mode, personal contact, and intervention intensity and duration. Obes Rev. 2017; 18:450–459. PMID: 28187246.38. Allen JK, Stephens J, Dennison Himmelfarb CR, Stewart KJ, Hauck S. Randomized controlled pilot study testing use of smartphone technology for obesity treatment. J Obes. 2013; 2013:151597. PMID: 24392223.39. Carter MC, Burley VJ, Nykjaer C, Cade JE. Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial. J Med Internet Res. 2013; 15:e32. PMID: 23587561.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Website and Mobile Application-Based Interventions for Adolescents and Young Adults with Depression: A Systematic Review and Meta-Analysis
- The Perception of Laymen and Experts Toward Mobile Applications for Self-monitoring of Diet Based on in-depth Interviews and Focus Group Interviews
- Effectiveness of a mobile health intervention on weight loss and dietary behavior changes among employees with overweight and obesity: a 12-week intervention study investigating the role of engagement
- Effects of Short-term Mobile Application Use on Weight Reduction for Patients with Type 2 Diabetes
- Effects of Mobile Phone-Based App Learning Compared to Computer-Based Web Learning on Nursing Students: Pilot Randomized Controlled Trial