J Liver Cancer.
2023 Sep;23(2):362-376. 10.17998/jlc.2023.08.03.
The efficacy of treatment for hepatocellular carcinoma in elderly patients
- Affiliations
-
- 1Department of Internal Medicine, Ewha Womans University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Catholic Kwandong University International St. Mary's Hospital, Catholic Kwandong University College of Medicine, Incheon, Korea
- 3Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
- 5Division of Gastroenterology and Hepatology, Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea
- 6Department of Internal Medicine, Uijeongbu Eulji Medical Center, Eulji University, Eulji University School of Medicine, Uijeongbu, Korea
- 7Department of Gastroenterology, Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 8Center for Liver and Pancreatobiliary Cancer, National Cancer Center, Goyang, Korea
- 9The Catholic University Liver Research Center, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 10Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 11Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 12Yonsei Liver Cancer Center, Severance Hospital, Seoul, Korea
- KMID: 2546416
- DOI: http://doi.org/10.17998/jlc.2023.08.03
Abstract
- Background
/Aim: Despite the increasing proportion of elderly patients with hepatocellular carcinoma (HCC) over time, treatment efficacy in this population is not well established.
Methods
Data collected from the Korean Primary Liver Cancer Registry, a representative cohort of patients newly diagnosed with HCC in Korea between 2008 and 2017, were analyzed. Overall survival (OS) according to tumor stage and treatment modality was compared between elderly and non-elderly patients with HCC.
Results
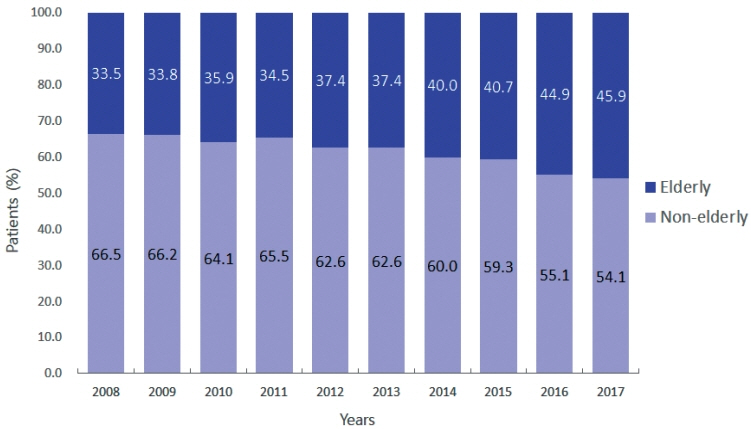
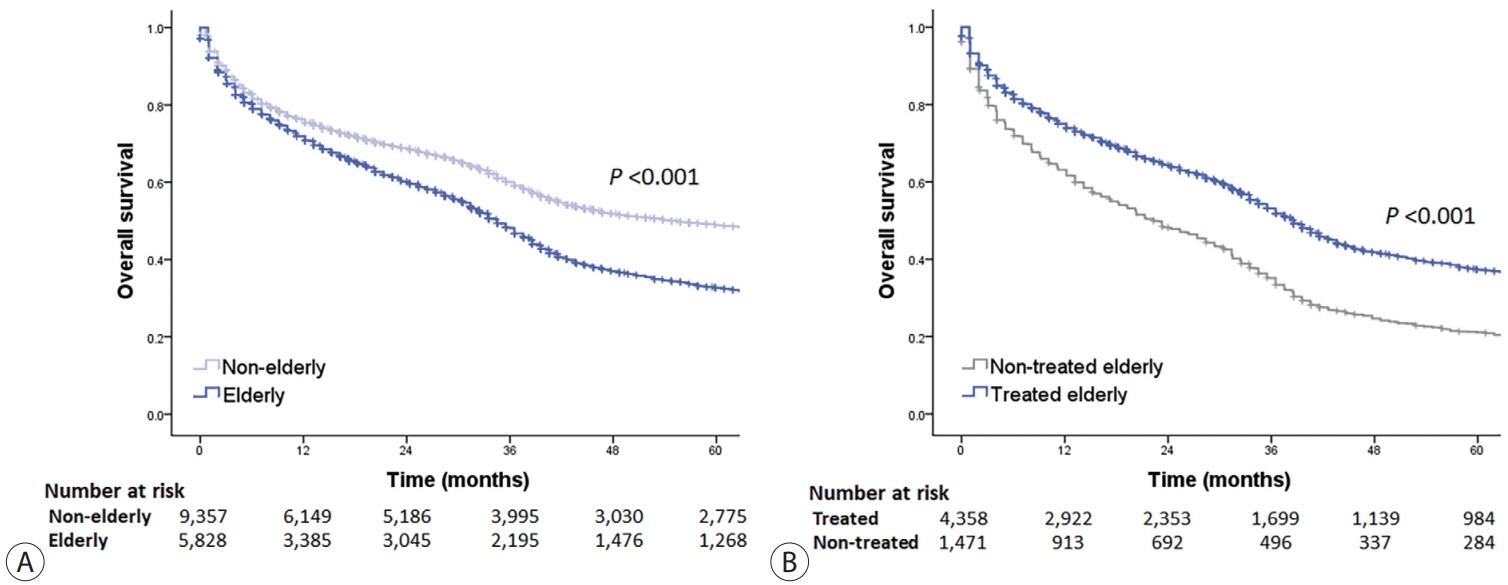
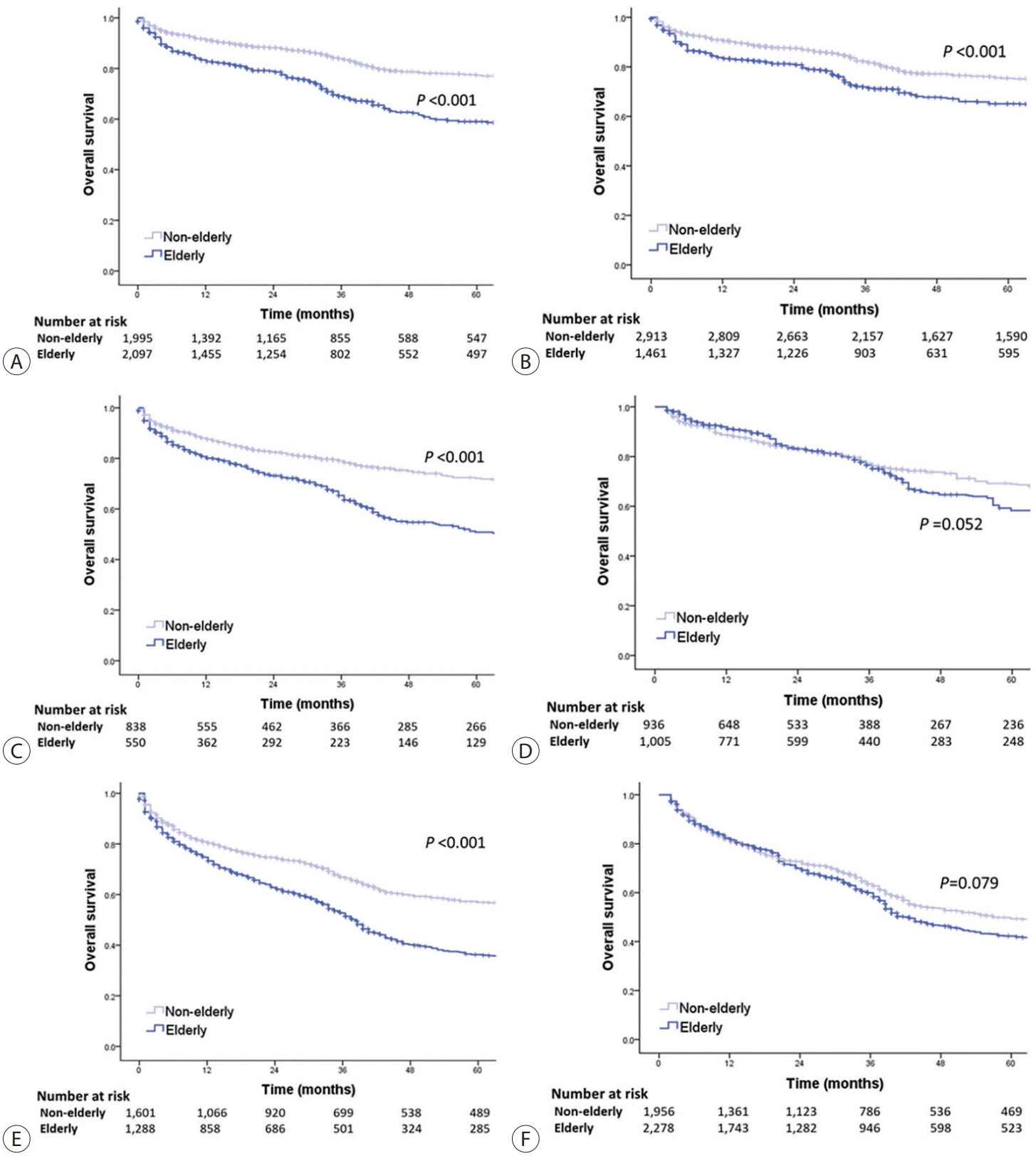
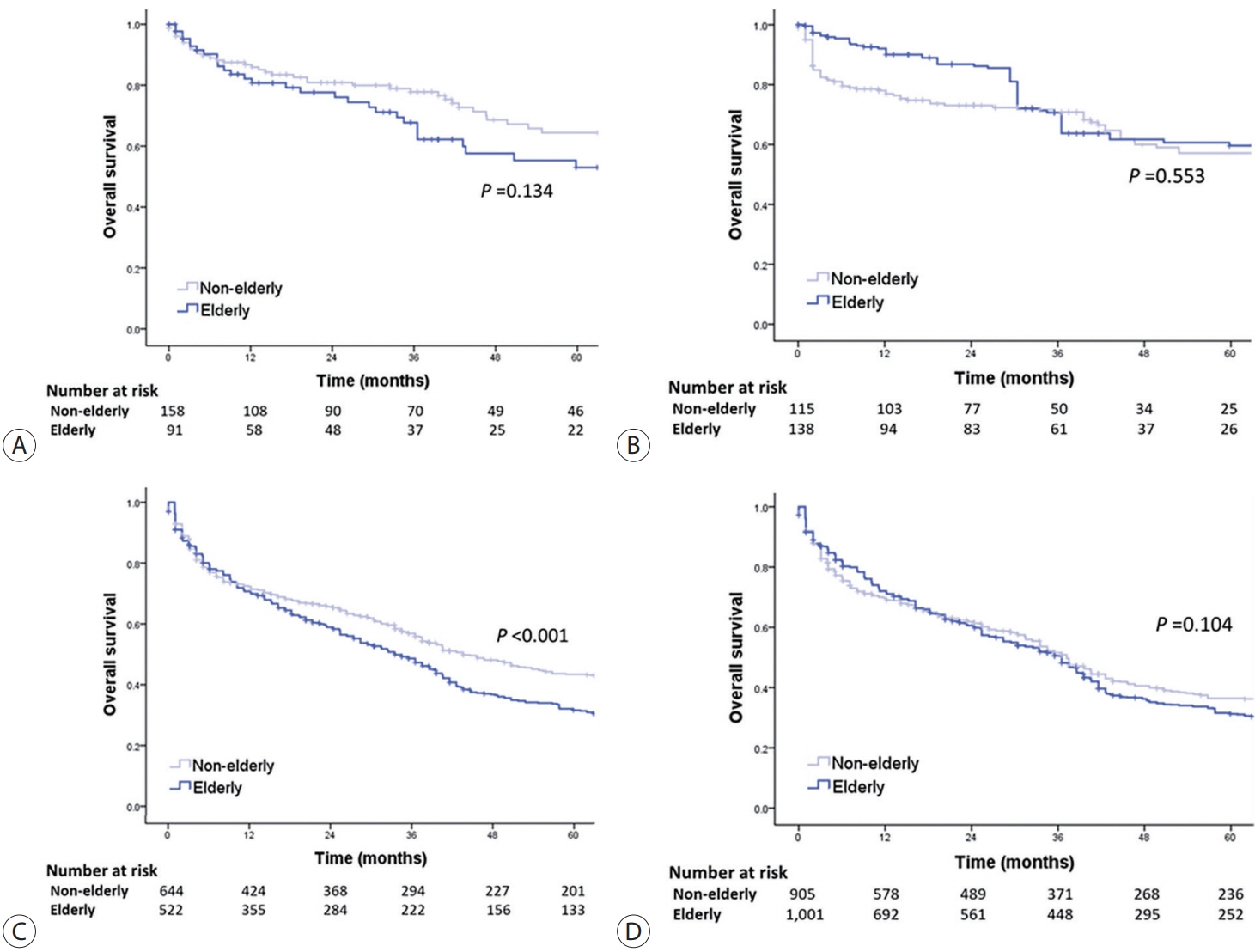
Among 15,186 study patients, 5,829 (38.4%) were elderly. A larger proportion of elderly patients did not receive any treatment for HCC than non-elderly patients (25.2% vs. 16.7%). However, OS was significantly better in elderly patients who received treatment compared to those who did not (median, 38.6 vs. 22.3 months; P<0.001). In early-stage HCC, surgery yielded significantly lower OS in elderly patients compared to non-elderly patients (median, 97.4 vs. 138.0 months; P<0.001), however, local ablation (median, 82.2 vs. 105.5 months) and transarterial therapy (median, 42.6 vs. 56.9 months) each provided comparable OS between the two groups after inverse probability of treatment weighting (IPTW) analysis (all P>0.05). After IPTW, in intermediate-stage HCC, surgery (median, 66.0 vs. 90.3 months) and transarterial therapy (median, 36.5 vs. 37.2 months), and in advanced-stage HCC, transarterial (median, 25.3 vs. 26.3 months) and systemic therapy (median, 25.3 vs. 26.3 months) yielded comparable OS between the elderly and non-elderly HCC patients (all P>0.05).
Conclusions
Personalized treatments tailored to individual patients can improve the prognosis of elderly patients with HCC to a level comparable to that of non-elderly patients.
Figure
Reference
-
References
1. Statistics Korea. Annual report on the causes of death statistics 2019 [Internet]. Daejeon (KR): Statistics Korea; [cited 2021 Feb 1]. Available from: https://kostat.go.kr/board.es?mid=a10301060100&bid=218&act=view&list_no=385219&tag=&nPage=1&ref_bid=.2. National Cancer Center. Annual report of cancer statistics in Korea in 2018 [Internet]. Goyang (KR): National Cancer Center; [cited 2021 Feb 1]. Available from: https://ncc.re.kr/cancerStatsView.ncc?bbsnum=558&searchKey=total&searchValue=&pageNum=1.3. Kim DY, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer. 2012; 1:2–14.4. Cho Y, Kim BH, Park JW. Overview of Asian clinical practice guidelines for the management of hepatocellular carcinoma: an Asian perspective comparison. Clin Mol Hepatol. 2023; 29:252–262.5. Statistics Korea. Population projections and summary indicators (Korea) [Internet]. Daejeon (KR): Statistics Korea; [cited 2023 May 1]. Available from: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1BPA002&conn_path=I2&language=en.6. Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018; 142:2471–2477.7. Chon YE, Park SY, Hong HP, Son D, Lee J, Yoon E, et al. Hepatocellular carcinoma incidence is decreasing in Korea but increasing in the very elderly. Clin Mol Hepatol. 2023; 29:120–134.8. Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 2022; 28:276–331.9. Macias RIR, Monte MJ, Serrano MA, González-Santiago JM, Martín-Arribas I, Simão AL, et al. Impact of aging on primary liver cancer: epidemiology, pathogenesis and therapeutics. Aging (Albany NY). 2021; 13:23416–23434.10. Seo JH, Kim DH, Cho E, Jun CH, Park SY, Cho SB, et al. Characteristics and outcomes of extreme elderly patients with hepatocellular carcinoma in South Korea. In Vivo. 2019; 33:145–154.11. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022; 28:583–705.12. Tabrizian P, Saberi B, Holzner ML, Rocha C, Kyung Jung Y, Myers B, et al. Outcomes of transplantation for HBV- vs. HCV-related HCC: impact of DAA HCV therapy in a national analysis of >20,000 patients. HPB (Oxford). 2022; 24:1082–1090.13. Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009; 136:138–148.14. Sohn W, Paik YH, Cho JY, Lim HY, Ahn JM, Sinn DH, et al. Sorafenib therapy for hepatocellular carcinoma with extrahepatic spread: treatment outcome and prognostic factors. J Hepatol. 2015; 62:1112–1121.15. Nguyen VT, Amin J, Law MG, Dore GJ. Predictors and survival in hepatitis B-related hepatocellular carcinoma in New South Wales, Australia. J Gastroenterol Hepatol. 2009; 24:436–442.16. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018; 68:723–750.17. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.18. Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012; 107:569–577. quiz 578.19. Takahashi H, Mizuta T, Kawazoe S, Eguchi Y, Kawaguchi Y, Otuka T, et al. Efficacy and safety of radiofrequency ablation for elderly hepatocellular carcinoma patients. Hepatol Res. 2010; 40:997–1005.20. Hiraoka A, Michitaka K, Horiike N, Hidaka S, Uehara T, Ichikawa S, et al. Radiofrequency ablation therapy for hepatocellular carcinoma in elderly patients. J Gastroenterol Hepatol. 2010; 25:403–407.21. Kishida N, Hibi T, Itano O, Okabayashi K, Shinoda M, Kitago M, et al. Validation of hepatectomy for elderly patients with hepatocellular carcinoma. Ann Surg Oncol. 2015; 22:3094–3101.22. Wu FH, Shen CH, Luo SC, Hwang JI, Chao WS, Yeh HZ, et al. Liver resection for hepatocellular carcinoma in oldest old patients. World J Surg Oncol. 2019; 17:1.23. Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002; 359:1734–1739.24. Nishikawa H, Kita R, Kimura T, Ohara Y, Takeda H, Sakamoto A, et al. Transcatheter arterial chemoembolization for intermediatestage hepatocellular carcinoma: clinical outcome and safety in elderly patients. J Cancer. 2014; 5:590–597.25. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.26. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 noninferiority trial. Lancet. 2018; 391:1163–1173.27. Kim Y, Lee JS, Lee HW, Kim BK, Park JY, Kim DY, et al. Sorafenib versus nivolumab after lenvatinib treatment failure in patients with advanced hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2023; 35:191–197.28. Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012; 82:2004–2011.29. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–1905.30. Vithayathil M, D'Alessio A, Fulgenzi CAM, Nishida N, Schönlein M, von Felden J, et al. Impact of older age in patients receiving atezolizumab and bevacizumab for hepatocellular carcinoma. Liver Int. 2022; 42:2538–2547.31. Dhondt E, Lambert B, Hermie L, Huyck L, Vanlangenhove P, Geerts A, et al. 90Y radioembolization versus drug-eluting bead chemoembolization for unresectable hepatocellular carcinoma: results from the TRACE phase II randomized controlled trial. Radiology. 2022; 303:699–710.32. Cho YY, Lee M, Kim HC, Chung JW, Kim YH, Gwak GY, et al. Radioembolization is a safe and effective treatment for hepatocellular carcinoma with portal vein thrombosis: a propensity score analysis. PLoS One. 2016; 11:e0154986.33. Golfieri R, Bilbao JI, Carpanese L, Cianni R, Gasparini D, Ezziddin S, et al. Comparison of the survival and tolerability of radioembolization in elderly vs. younger patients with unresectable hepatocellular carcinoma. J Hepatol. 2013; 59:753–761.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment and Surveillance of Hepatocellular Carcinoma in Elderly Patients
- Hepatocellular Carcinoma Arising from Hepatocellular Adenoma in an Elderly Male Patient
- The Natural Course of Hepatocellular Carcinoma in a Super-Elderly Patient
- The emerging age-pattern changes of patients with hepatocellular carcinoma in Korea
- Metastatic Omental Hepatocellular Carcinoma: Two Cases Report