J Liver Cancer.
2023 Sep;23(2):341-349. 10.17998/jlc.2023.04.30.
Isolation and characterization of cancer-associated fibroblasts in the tumor microenvironment of hepatocellular carcinoma
- Affiliations
-
- 1The Catholic University Liver Research Center and POSTECH-Catholic Biomedical Engineering Institute, College of Medicine, The Catholic University Korea, Seoul, Korea
- 2Division of Gastroenterology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Division of Chronic Viral Disease Research, Center for Emerging Virus Research, National Institute of Infectious Diseases, Cheongju, Korea
- 4Department of Surgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2546414
- DOI: http://doi.org/10.17998/jlc.2023.04.30
Abstract
- Background
/Aim: Cancer-associated fibroblasts (CAFs) play an immunosuppressive role in the tumor microenvironment (TME) of human cancers; however, their characteristics and role in hepatocellular carcinoma (HCC) remain to be elucidated.
Methods
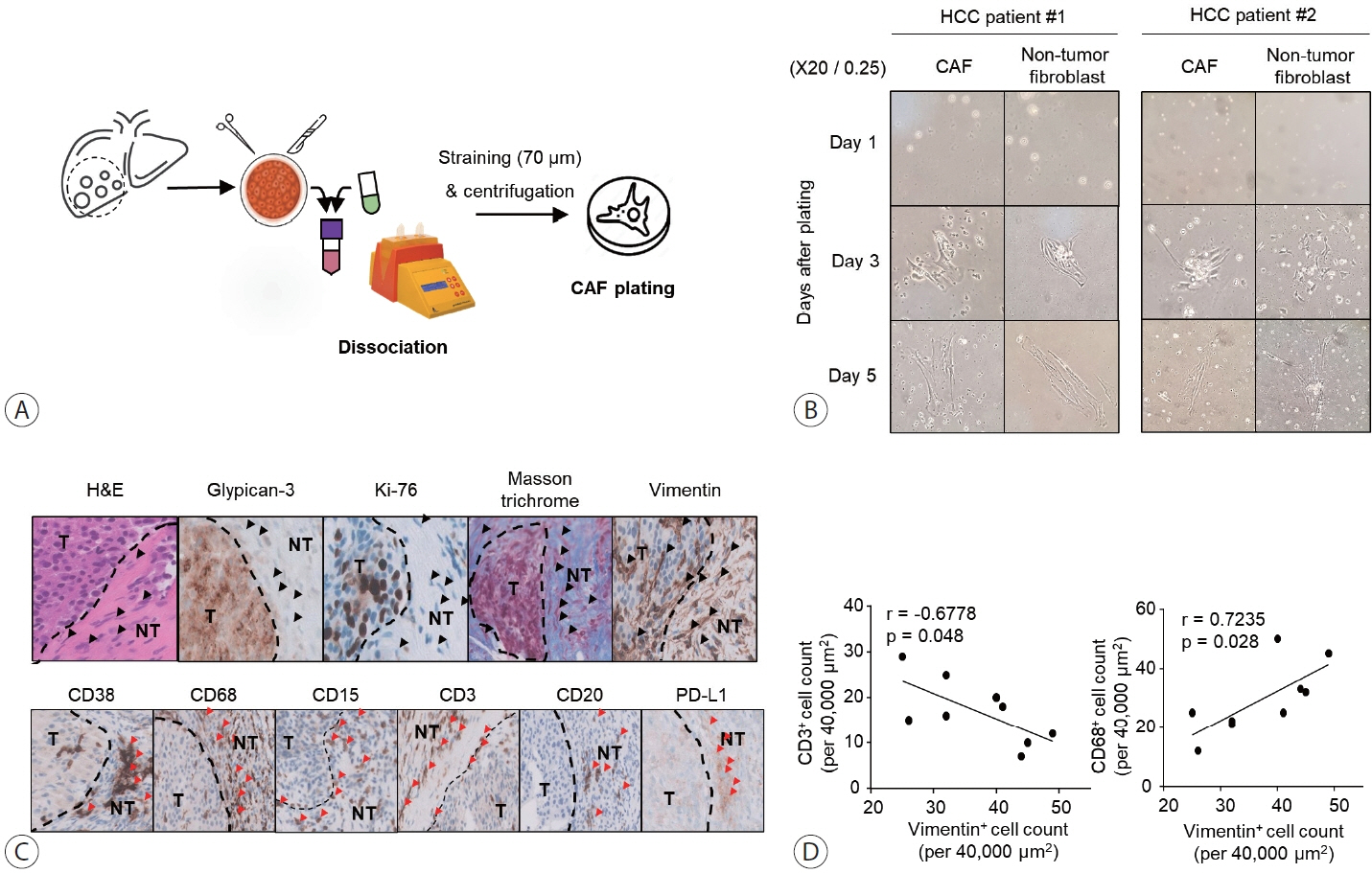
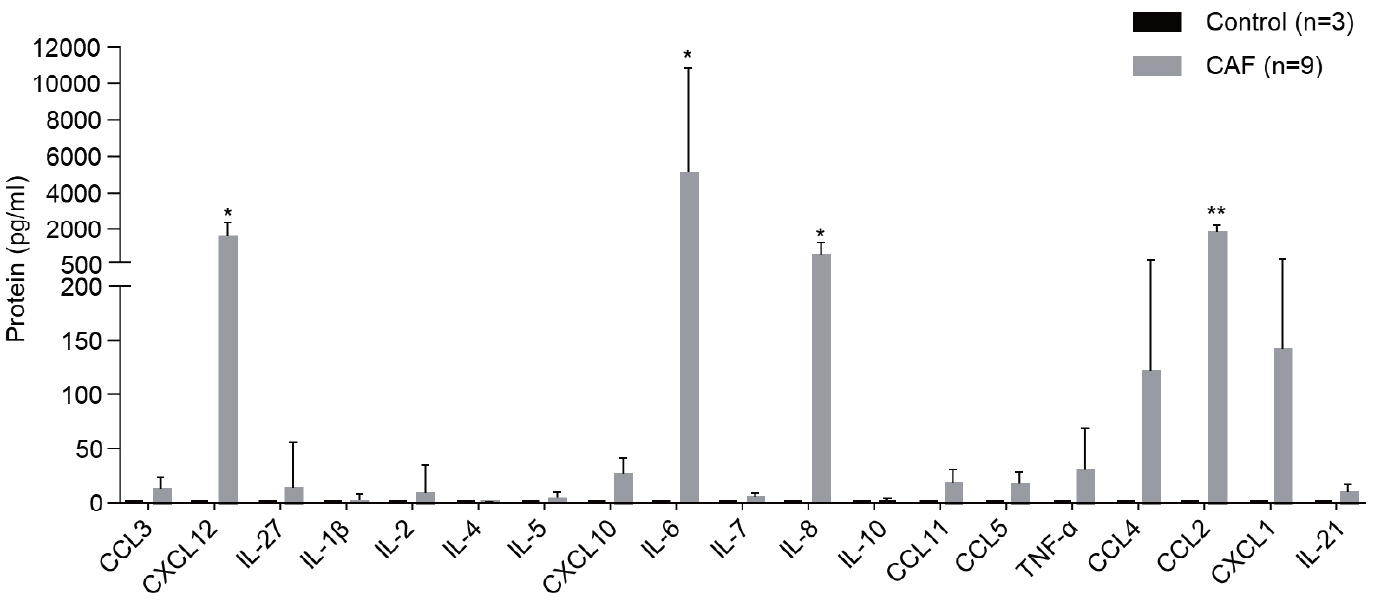
Nine tumor and surrounding liver tissue samples from patients with HCC who underwent surgery were used to isolate patient-derived CAFs. Cell morphology was observed using an optical microscope after culture, and cell phenotypes were evaluated using flow cytometry and immunoblotting. Cytokines secreted by CAFs into culture medium were quantified using a multiplex cytokine assay.
Results
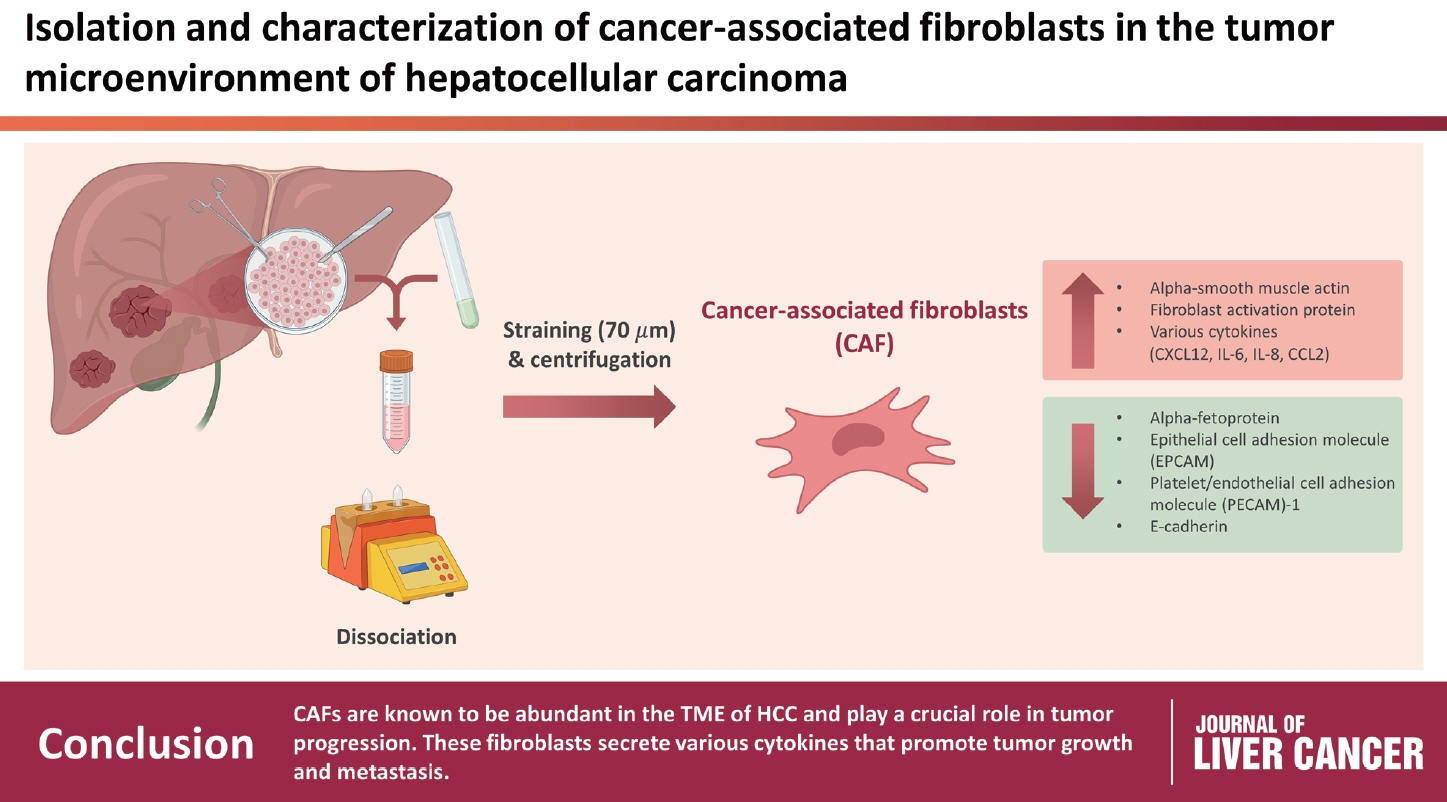
CAFs were abundant in the TME of HCC and were adjacent to immune cells. After culture, the CAFs and non-tumor fibroblasts exhibited spindle shapes. We observed a robust expression of alpha-smooth muscle actin and fibroblast activation protein in CAFs, whereas alpha-fetoprotein, epithelial cell adhesion molecule, platelet/endothelial cell adhesion molecule-1, and E-cadherin were not expressed in CAFs. Furthermore, CAFs showed high secretion of various cytokines, namely C-X-C motif chemokine ligand 12, interleukin (IL)-6, IL-8, and C-C motif chemokine ligand 2.
Conclusions
CAFs are abundant in the TME of HCC and play a crucial role in tumor progression. These fibroblasts secrete cytokines that promote tumor growth and metastasis.
Figure
Reference
-
References
1. Sung PS, Choi MH, Yang H, Lee SK, Chun HJ, Jang JW, et al. Diffusion-weighted magnetic resonance imaging in hepatocellular carcinoma as a predictor of a response to cisplatin-based hepatic arterial infusion chemotherapy. Front Oncol. 2020; 10:600233.2. Park SH, Heo S, Kim B, Lee J, Choi HJ, Sung PS, et al. Targetoid primary liver malignancy in chronic liver disease: prediction of postoperative survival using preoperative MRI findings and clinical factors. Korean J Radiol. 2023; 24:190–203.3. Feng MY, Chan LL, Chan SL. Drug treatment for advanced hepatocellular carcinoma: first-line and beyond. Curr Oncol. 2022; 29:5489–5507.4. Mizukoshi E, Kaneko S. Immune cell therapy for hepatocellular carcinoma. J Hematol Oncol. 2019; 12:52.5. Zhang Q, Lou Y, Bai XL, Liang TB. Intratumoral heterogeneity of hepatocellular carcinoma: from single-cell to population-based studies. World J Gastroenterol. 2020; 26:3720–3736.6. Sung PS, Jang JW, Lee J, Lee SK, Lee HL, Yang H, et al. Real-world outcomes of nivolumab in patients with unresectable hepatocellular carcinoma in an endemic area of hepatitis B virus infection. Front Oncol. 2020; 10:1043.7. Sas Z, Cendrowicz E, Weinhäuser I, Rygiel TP. Tumor microenvironment of hepatocellular carcinoma: challenges and opportunities for new treatment options. Int J Mol Sci. 2022; 23:3778.8. Park DJ, Sung PS, Lee GW, Cho S, Kim SM, Kang BY, et al. Preferential expression of programmed death ligand 1 protein in tumorassociated macrophages and its potential role in immunotherapy for hepatocellular carcinoma. Int J Mol Sci. 2021; 22:4710.9. Sung PS. Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma. Clin Mol Hepatol. 2022; 28:333–350.10. Zampaglione L, Ferrari J, Pedica F, Goossens N. HCC in metabolic syndrome: current concepts and future directions. Hepatoma Res. 2021; 7:55.11. Eskandari-Malayeri F, Rezaei M. Immune checkpoint inhibitors as mediators for immunosuppression by cancer-associated fibroblasts: a comprehensive review. Front Immunol. 2022; 13:996145.12. Sung PS, Lee IK, Roh PR, Kang MW, Ahn J, Yoon SK. Blood-based biomarkers for immune-based therapy in advanced HCC: promising but a long way to go. Front Oncol. 2022; 12:1028728.13. Loh JJ, Ma S. The role of cancer-associated fibroblast as a dynamic player in mediating cancer stemness in the tumor microenvironment. Front Cell Dev Biol. 2021; 9:727640.14. Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019; 12:86.15. Zhang T, Ren Y, Yang P, Wang J, Zhou H. Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. Cell Death Dis. 2022; 13:897.16. Foster DS, Januszyk M, Delitto D, Yost KE, Griffin M, Guo J, et al. Multiomic analysis reveals conservation of cancer-associated fibroblast phenotypes across species and tissue of origin. Cancer Cell. 2022; 40:1392–1406.e7.17. Norton J, Foster D, Chinta M, Titan A, Longaker M. Pancreatic cancer associated fibroblasts (CAF): under-explored target for pancreatic cancer treatment. Cancers (Basel). 2020; 12:1347.18. Eun JW, Yoon JH, Ahn HR, Kim S, Kim YB, Lim SB, et al. Cancer-associated fibroblast-derived secreted phosphoprotein 1 contributes to resistance of hepatocellular carcinoma to sorafenib and lenvatinib. Cancer Commun (Lond). 2023; 43:455–479.19. Simon T, Salhia B. Cancer-associated fibroblast subpopulations with diverse and dynamic roles in the tumor microenvironment. Mol Cancer Res. 2022; 20:183–192.20. Toledo B, Picon-Ruiz M, Marchal JA, Perán M. Dual role of fibroblasts educated by tumour in cancer behavior and therapeutic perspectives. Int J Mol Sci. 2022; 23:15576.21. Desbois M, Wang Y. Cancer-associated fibroblasts: key players in shaping the tumor immune microenvironment. Immunol Rev. 2021; 302:241–258.22. Feng B, Wu J, Shen B, Jiang F, Feng J. Cancer-associated fibroblasts and resistance to anticancer therapies: status, mechanisms, and countermeasures. Cancer Cell Int. 2022; 22:166.23. Sullivan KM, Jiang X, Guha P, Lausted C, Carter JA, Hsu C, et al. Blockade of interleukin 10 potentiates antitumour immune function in human colorectal cancer liver metastases. Gut. 2023; 72:325–337.24. Raskov H, Orhan A, Gaggar S, Gögenur I. Cancer-associated fibroblasts and tumor-associated macrophages in cancer and cancer immunotherapy. Front Oncol. 2021; 11:668731.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tumor Cells and Cancer-Associated Fibroblasts: A Synergistic Crosstalk to Promote Thyroid Cancer
- Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma
- Role of Periostin in Hepatocellular Carcinoma: The Importance of Tumor Microenvironment
- Lymphopenia predicts reduced survival in canine hepatocellular carcinoma
- Interactions between Immune Cells and Tumor Cells