J Liver Cancer.
2023 Sep;23(2):330-340. 10.17998/jlc.2023.04.14.
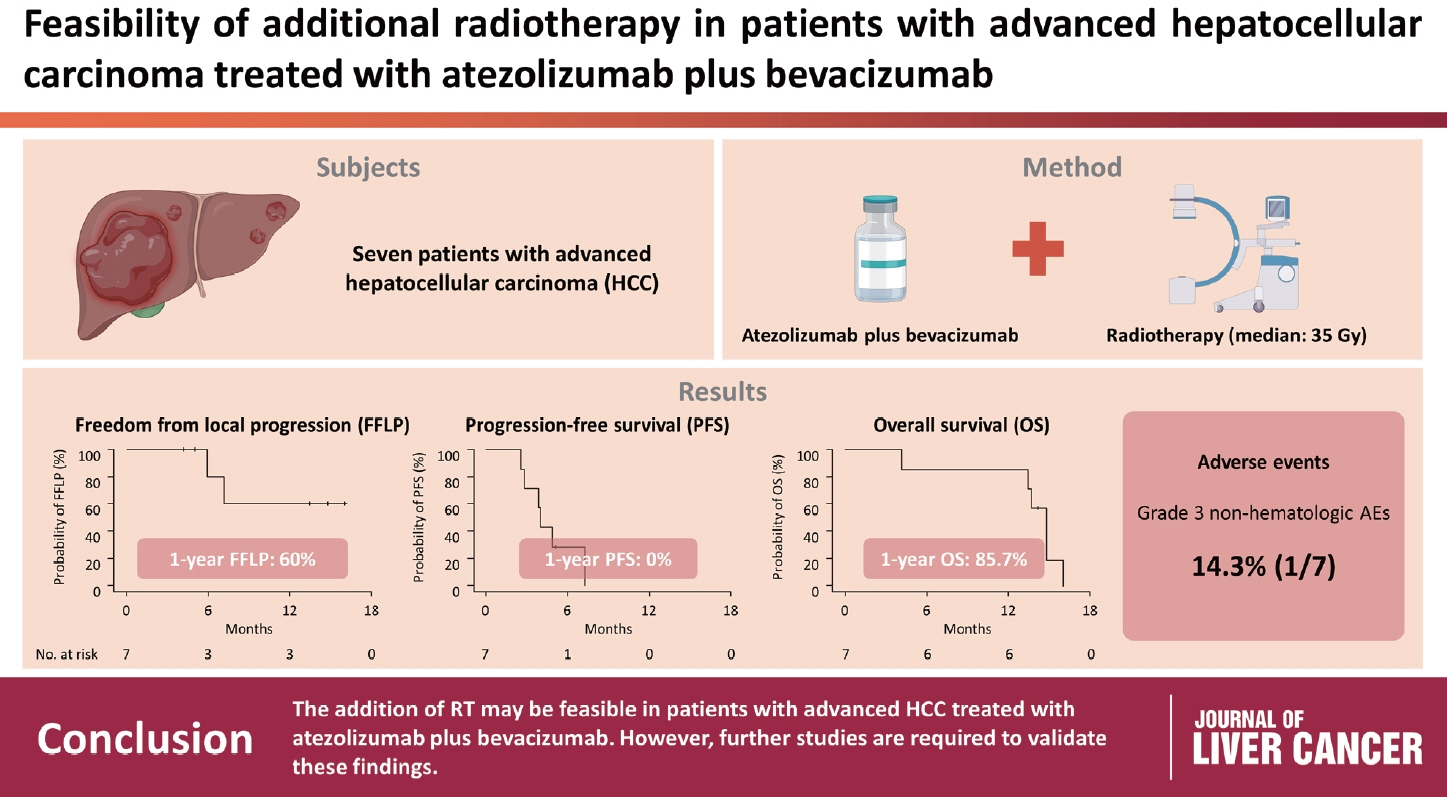
Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab
- Affiliations
-
- 1Center for Liver and Pancreatobiliary Cancer, National Cancer Center, Goyang, Korea
- 2Proton Therapy Center, National Cancer Center, Goyang, Korea
- KMID: 2546413
- DOI: http://doi.org/10.17998/jlc.2023.04.14
Abstract
- Background
/Aim: Radiotherapy (RT) is an effective local treatment for hepatocellular carcinoma (HCC). However, whether additional RT is safe and effective in patients with advanced HCC receiving atezolizumab plus bevacizumab remains unclear. This retrospective cohort study aimed to evaluate the feasibility of additional RT in these patients.
Methods
Between March and October 2021, we retrospectively analyzed seven patients with advanced HCC who received RT during treatment with atezolizumab plus bevacizumab. The median prescribed RT dose was 35 Gy (range, 33–66). Freedom from local progression (FFLP), progression-free survival (PFS), and overall survival (OS) after RT were analyzed.
Results
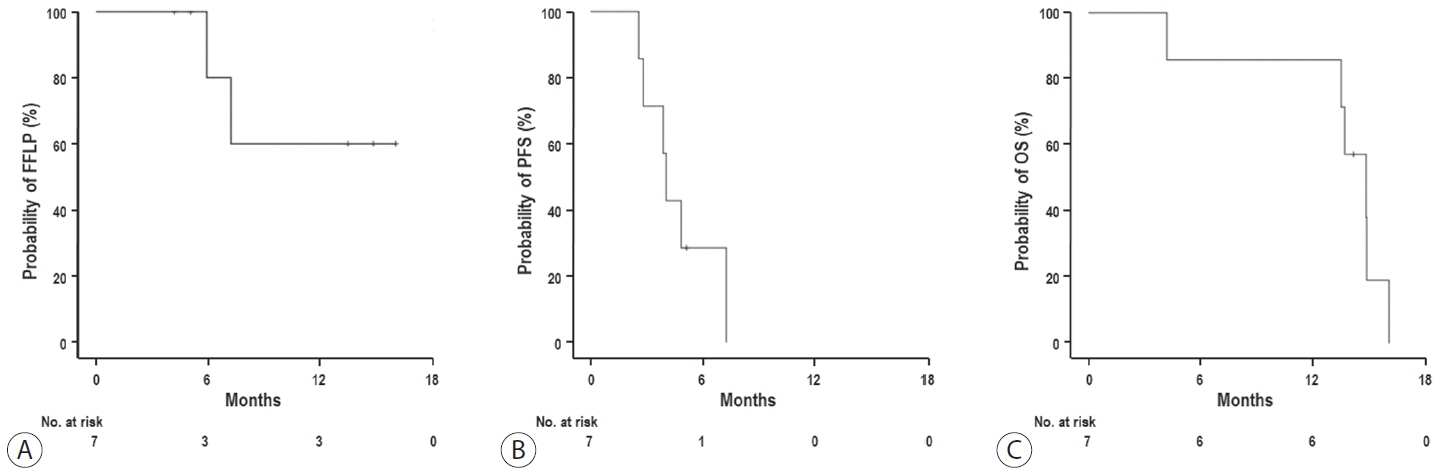
The median follow-up duration after RT was 14.2 months (range, 10.0–18.6). Of the seven patients, disease progression was noted in six (85.7%), the sites of disease progression were local in two (28.6%), intrahepatic in four (57.1%), and extrahepatic in four (57.1%). The median time of FFLP was not reached, and PFS and OS times were 4.0 (95% confidence interval [CI], 3.6–4.5) and 14.8% (95% CI, 12.5–17.2) months, respectively. The 1-year FFLP, PFS, and OS rates were 60% (95% CI, 43.8–76.2), 0%, and 85.7% (95% CI, 75.9–95.5), respectively. Grade 3 or higher hematologic adverse events (AEs) were not observed, but grade 3 nonhematologic AEs unrelated to RT were observed in one patient.
Conclusions
The addition of RT may be feasible in patients with advanced HCC treated with atezolizumab plus bevacizumab. However, further studies are required to validate these findings.
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249.2. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015; 35:2155–2166.3. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.4. Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019; 13:227–299.5. Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021; 10:181–223.6. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018; 68:723–750.7. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.8. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.9. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 noninferiority trial. Lancet. 2018; 391:1163–1173.10. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022; 76:862–873.11. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–1905.12. Manzar GS, De BS, Abana CO, Lee SS, Javle M, Kaseb AO, et al. Outcomes and toxicities of modern combined modality therapy with atezolizumab plus bevacizumab and radiation therapy for hepatocellular carcinoma. Cancers (Basel). 2022; 14:1901.13. Chiang CL, Chan ACY, Chiu KWH, Kong FS. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: a potential synergistic treatment strategy. Front Oncol. 2019; 9:1157.14. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014; 124:687–695.15. Kim KJ, Kim JH, Lee SJ, Lee EJ, Shin EC, Seong J. Radiation improves antitumor effect of immune checkpoint inhibitor in murine hepatocellular carcinoma model. Oncotarget. 2017; 8:41242–41255.16. Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One. 2016; 11:e0157164.17. Fukuda K, Okumura T, Abei M, Fukumitsu N, Ishige K, Mizumoto M, et al. Long-term outcomes of proton beam therapy in patients with previously untreated hepatocellular carcinoma. Cancer Sci. 2017; 108:497–503.18. Fukumitsu N, Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Abei M, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009; 74:831–836.19. Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, et al. Multi-institutional phase ii study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2016; 34:460–468.20. Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005; 23:1839–1846.21. Kim DY, Park JW, Kim TH, Kim BH, Moon SH, Kim SS, et al. Riskadapted simultaneous integrated boost-proton beam therapy (SIB-PBT) for advanced hepatocellular carcinoma with tumour vascular thrombosis. Radiother Oncol. 2017; 122:122–129.22. Kim TH, Kim BH, Park JW, Cho YR, Koh YH, Chun JW, et al. Proton beam therapy for treatment-naïve hepatocellular carcinoma and prognostic significance of albumin-bilirubin (ALBI) grade. Cancers (Basel). 2022; 14:4445.23. Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B, et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: a randomized phase III trial. J Hepatol. 2021; 74:603–612.24. Kim TH, Park JW, Kim BH, Kim H, Moon SH, Kim SS, et al. Does risk-adapted proton beam therapy have a role as a complementary or alternative therapeutic option for hepatocellular carcinoma? Cancers (Basel). 2019; 11:230.25. Kim TH, Park JW, Kim BH, Oh ES, Youn SH, Moon SH, et al. Phase II study of hypofractionated proton beam therapy for hepatocellular carcinoma. Front Oncol. 2020; 10:542.26. Kim TH, Park JW, Kim YJ, Kim BH, Woo SM, Moon SH, et al. Phase I dose-escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Cancer Res Treat. 2015; 47:34–45.27. Kim Y, Park HC, Yoon SM, Kim TH, Lee J, Choi J, et al. Prognostic group stratification and nomogram for predicting overall survival in patients who received radiotherapy for abdominal lymph node metastasis from hepatocellular carcinoma: a multi-institutional retrospective study (KROG 15-02). Oncotarget. 2017; 8:94450–94461.28. Kim TH, Park JW, Kim YJ, Kim BH, Woo SM, Moon SH, et al. Simultaneous integrated boost-intensity modulated radiation therapy for inoperable hepatocellular carcinoma. Strahlenther Onkol. 2014; 190:882–890.29. Jung J, Yoon SM, Park HC, Nam TK, Seong J, Chie EK, et al. Radiotherapy for adrenal metastasis from hepatocellular carcinoma: a multi-institutional retrospective study (KROG 13-05). PLoS One. 2016; 11:e0152642.30. Lee SU, Park JW, Kim TH, Kim YJ, Woo SM, Koh YH, et al. Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol. 2014; 190:806–814.31. Cho Y, Kim BH, Kim TH, Koh YH, Park JW. Sorafenib combined with radiation therapy for advanced hepatocellular carcinoma with portal and hepatic vein invasion extending to the inferior vena cava: a complete response case according to modified RECIST criteria. J Liver Cancer. 2022; 22:63–68.32. Cho Y, Kim BH, Kim TH, Koh YH, Park JW. A case of successful surgical treatment for peritoneal seeding of hepatocellular carcinoma after radiotherapy and atezolizumab plus bevacizumab combination treatment. J Liver Cancer. 2023; 23:206–212.33. Lee A, Lee J, Yang H, Sung SY, Jeon CH, Kim SH, et al. Multidisciplinary treatment with immune checkpoint inhibitors for advanced stage hepatocellular carcinoma. J Liver Cancer. 2022; 22:75–83.34. Kim YT, Kim J, Seong J. Favorable response of hepatocellular carcinoma with portal vein tumor thrombosis after radiotherapy combined with atezolizumab plus bevacizumab. J Liver Cancer. 2023; 23:225–229.35. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual, 8th ed. Berlin: Springer;2017.36. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015; 33:550–558.37. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter regarding “Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab”
- Favorable response of hepatocellular carcinoma with portal vein tumor thrombosis after radiotherapy combined with atezolizumab plus bevacizumab
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report
- A case of nearly complete response in hepatocellular carcinoma with disseminated lung metastasis by combination therapy of nivolumab and ipilimumab after treatment failure of atezolizumab plus bevacizumab
- Clinical significance of the discrepancy between radiological findings and biochemical responses in atezolizumab plus bevacizumab for hepatocellular carcinoma