Kosin Med J.
2023 Sep;38(3):219-223. 10.7180/kmj.23.105.
Central diabetes insipidus following COVID-19 mRNA vaccination: a case report
- Affiliations
-
- 1Department of Internal Medicine, Gyeongsang National University Hospital, Jinju, Korea
- 2Department of Internal Medicine, Gyeongsang National University School of Medicine, Jinju, Korea
- 3Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Korea
- 4Department of Internal Medicine, Gyeongsang National University Changwon Hospital, Changwon, Korea
- 5Department of Radiology, Gyeongsang National University Hospital, Jinju, Korea
- 6Department of Biochemistry, Gyeongsang National University School of Medicine Jinju, Korea
- KMID: 2546158
- DOI: http://doi.org/10.7180/kmj.23.105
Abstract
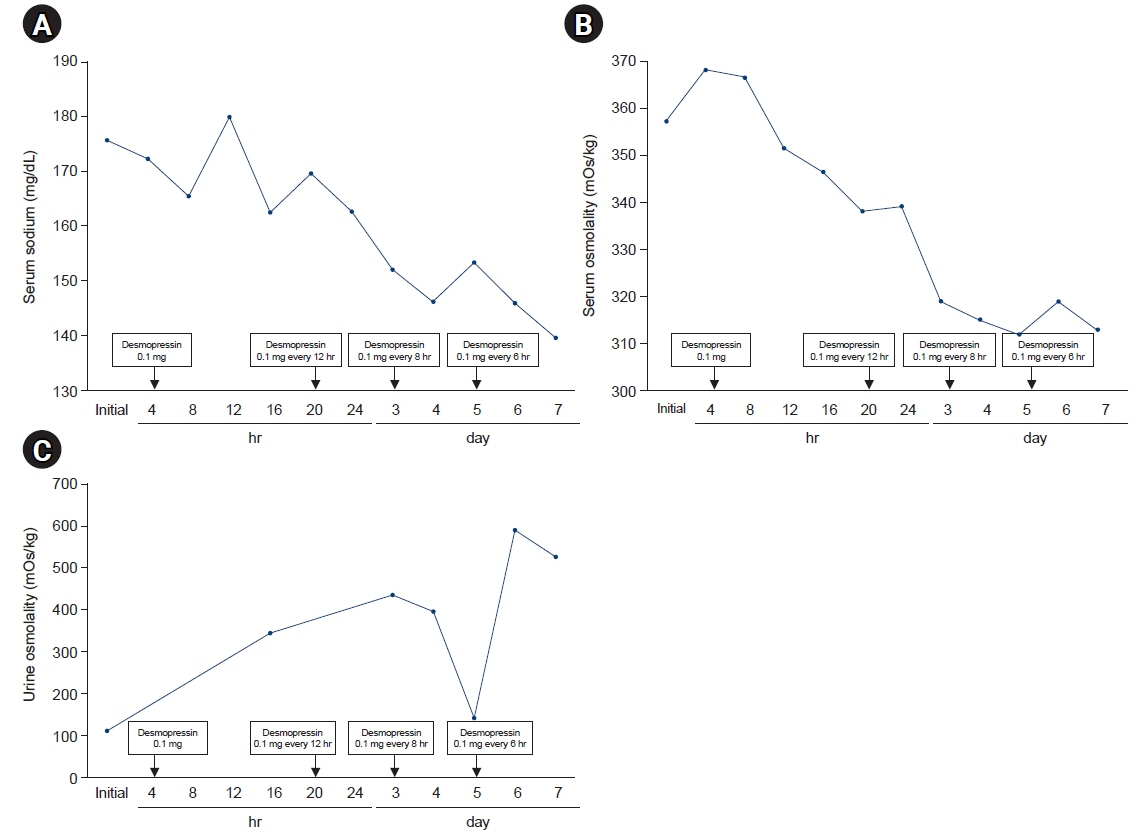
- The coronavirus disease 2019 (COVID-19) has been a major public health emergency worldwide. Vaccines were rapidly developed and approved to prevent the spread of viral infection. However, various side effects of the COVID-19 messenger RNA (mRNA) vaccines have been reported after their commercialization. A 24-year-old man visited our emergency department with polyuria and polydipsia that occurred after he received a COVID-19 mRNA vaccine 10 days beforehand. The initial laboratory findings showed very low urine osmolality with hyperosmolar hypernatremia. Based on these findings, diabetes insipidus was suspected, and sella magnetic resonance imaging showed an enlarged pituitary gland and the absence of posterior pituitary higher intensity. After 12 hours of using oral desmopressin acetate, urine volume decreased, and after 5 days of administration, serum electrolyte and serum osmolality improved. This case report of diabetes insipidus occurring after vaccination with the BNT162b2 mRNA COVID-19 vaccine is presented as a reminder that close monitoring is necessary for patients with polyuria and polydipsia after vaccination.
Keyword
Figure
Reference
-
References
1. U.S. Food and Drug Administration (FDA). Emergency use authorization: PfizerBioNTech COVID-19 vaccine [Internet]. U.S. FDA; c2021 [cited 2023 May 18]. https://www.fda.gov/vaccines-blood-biologics/coronavirus-covid-19-cber-regulated-biologics/pfizer-biontech-covid-19-vaccines.2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383:2603–15.
Article3. Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med. 2021; 385:2140–9.
Article4. Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021; 6:1202–6.
Article5. Krzywicka K, Heldner MR, Sanchez van Kammen M, van Haaps T, Hiltunen S, Silvis SM, et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: an analysis of cases notified to the European Medicines Agency. Eur J Neurol. 2021; 28:3656–62.
Article6. King ER, Towner E. A case of immune thrombocytopenia after BNT162b2 mRNA COVID-19 vaccination. Am J Case Rep. 2021; 22:e931478.
Article7. Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022; 165:386–401.
Article8. Garrahy A, Moran C, Thompson CJ. Diagnosis and management of central diabetes insipidus in adults. Clin Endocrinol (Oxf). 2019; 90:23–30.
Article9. Murvelashvili N, Tessnow A. A case of hypophysitis following immunization with the mRNA-1273 SARS-CoV-2 vaccine. J Investig Med High Impact Case Rep. 2021; 9:23247096211043386.
Article10. Bouca B, Roldao M, Bogalho P, Cerqueira L, Silva-Nunes J. Central diabetes insipidus following immunization with BNT162b2 mRNA COVID-19 vaccine: a case report. Front Endocrinol (Lausanne). 2022; 13:889074.11. Ankireddypalli AR, Chow LS, Radulescu A, Kawakami Y, Araki T. A case of hypophysitis associated with SARS-CoV-2 vaccination. AACE Clin Case Rep. 2022; 8:204–9.
Article12. Gubbi S, Hannah-Shmouni F, Verbalis JG, Koch CA. Hypophysitis: an update on the novel forms, diagnosis and management of disorders of pituitary inflammation. Best Pract Res Clin Endocrinol Metab. 2019; 33:101371.
Article13. Faje A. Hypophysitis: evaluation and management. Clin Diabetes Endocrinol. 2016; 2:15.
Article14. Taieb A, Mounira EE. Pilot findings on SARS-CoV-2 vaccine-induced pituitary diseases: a mini review from diagnosis to pathophysiology. Vaccines (Basel). 2022; 10:2004.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adult-Onset Type 1 Diabetes Development Following COVID-19 mRNA Vaccination
- Diabetes and COVID-19 Vaccination
- A Case of Central Diabetes Insipidus Associated with Brachycephaly
- Ipsilateral Radial Neuropathy after COVID-19 mRNA Vaccination in an Immunocompetent Young Man
- Morphea and Verruca Plana Complicated in Central Diabetes Insipidus