J Korean Neurosurg Soc.
2023 Sep;66(5):511-524. 10.3340/jkns.2023.0049.
Reparative, Neuroprotective and Anti-neurodegenerative Effects of Granulocyte Colony Stimulating Factor in Radiation-Induced Brain Injury Model
- Affiliations
-
- 1Department of Neurosurgery, Izmir Katip Celebi University Atatürk Training and Research Hospital, Izmir, Turkey
- 2Department of Radiation Oncology, Kartal City Hospital, Istanbul, Turkey
- 3Department of Radiology, Demiroğlu Bilim University, Istanbul, Turkey
- 4Department of Radiology, Istanbul Atlas University, Istanbul, Turkey
- 5Department of Radiation Oncology, Lutfi Kirdar Kartal Education and Research Hospital, Istanbul, Turkey
- 6Department of Histology and Embryology, Faculty of Medicine, Ege University, Izmir, Turkey
- 7Department of Neurosurgery, Faculty of Medicine, Dokuz Eylul University, Izmir, Turkey
- 8Department of Physiology, Katip Celebi University, Izmir, Turkey
- 9Department of Physiology, Demiroğlu Bilim University, Istanbul, Turkey
- KMID: 2545342
- DOI: http://doi.org/10.3340/jkns.2023.0049
Abstract
Objective
: This animal model aimed to compare the rat group that received brain irradiation and did not receive additional treatment (only saline) and the rat group that underwent brain irradiation and received Granulocyte colony stimulating factor (G-CSF) treatment. In addition, the effects of G-CSF on brain functions were examined by magnetic resonance (MR) imaging and histopathologically.
Methods
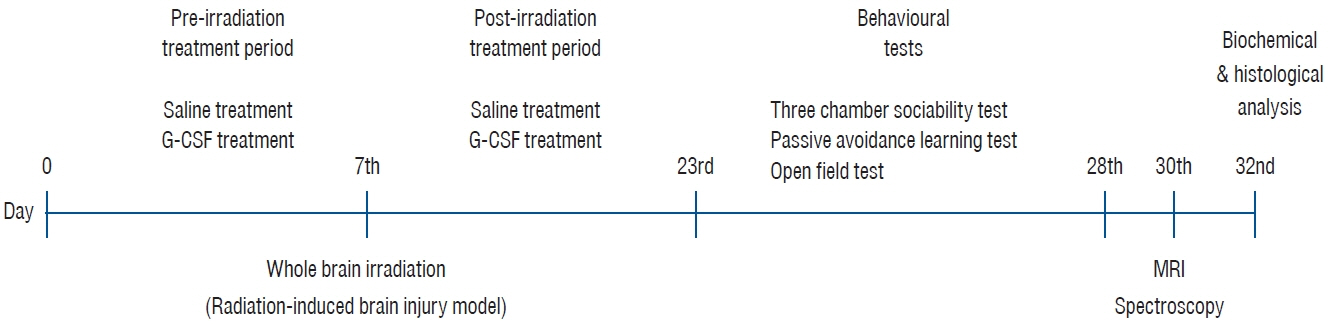
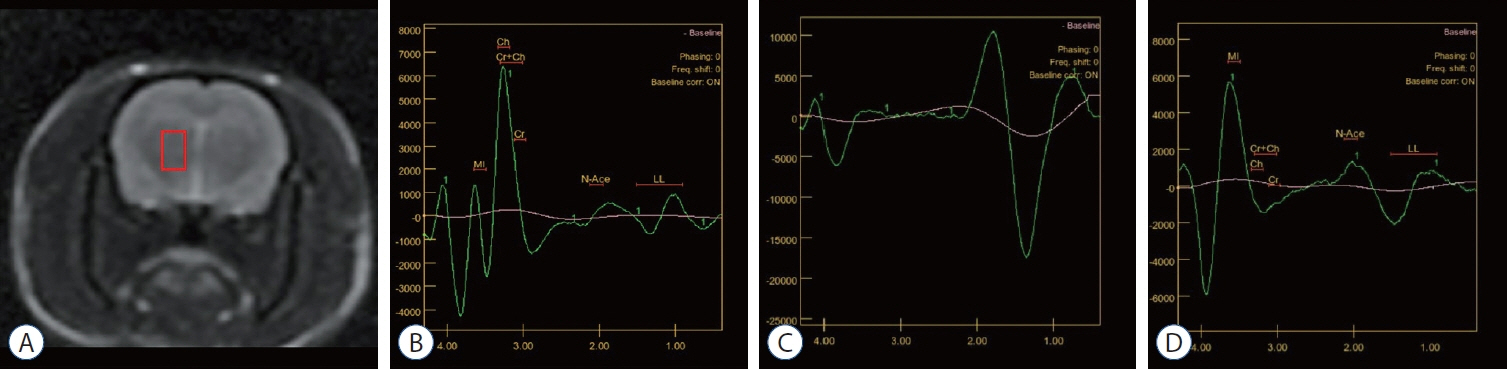
: This study used 24 female Wistar albino rats. Drug administration (saline or G-CSF) was started at the beginning of the study and continued for 15 days after whole-brain radiotherapy (WBRT). WBRT was given on day 7 of the start of the study. At the end of 15 days, the behavioral tests, including the three-chamber sociability test, open field test, and passive avoidance learning test, were done. After the behavioral test, the animals performed the MR spectroscopy procedure. At the end of the study, cervical dislocation was applied to all animals.
Results
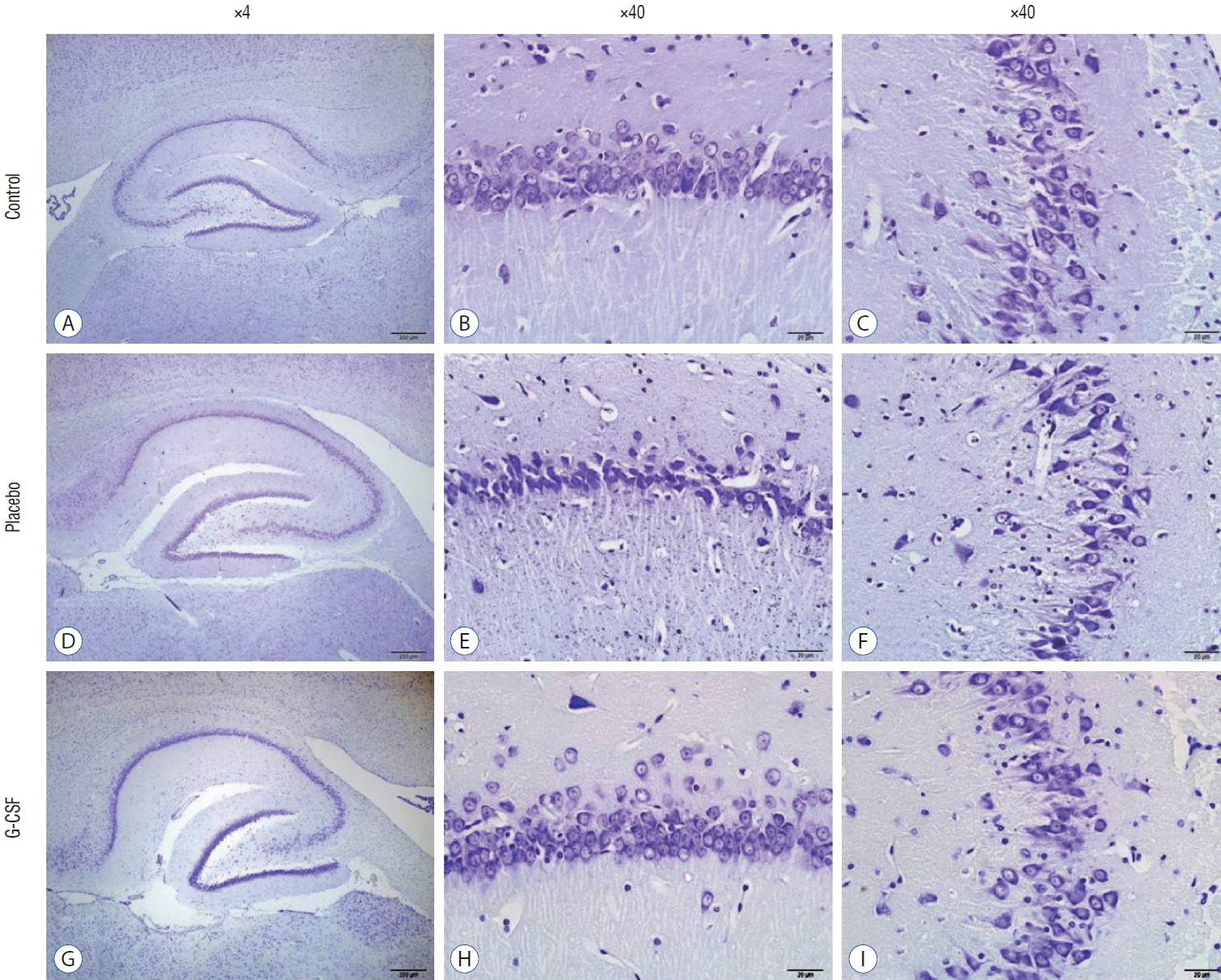
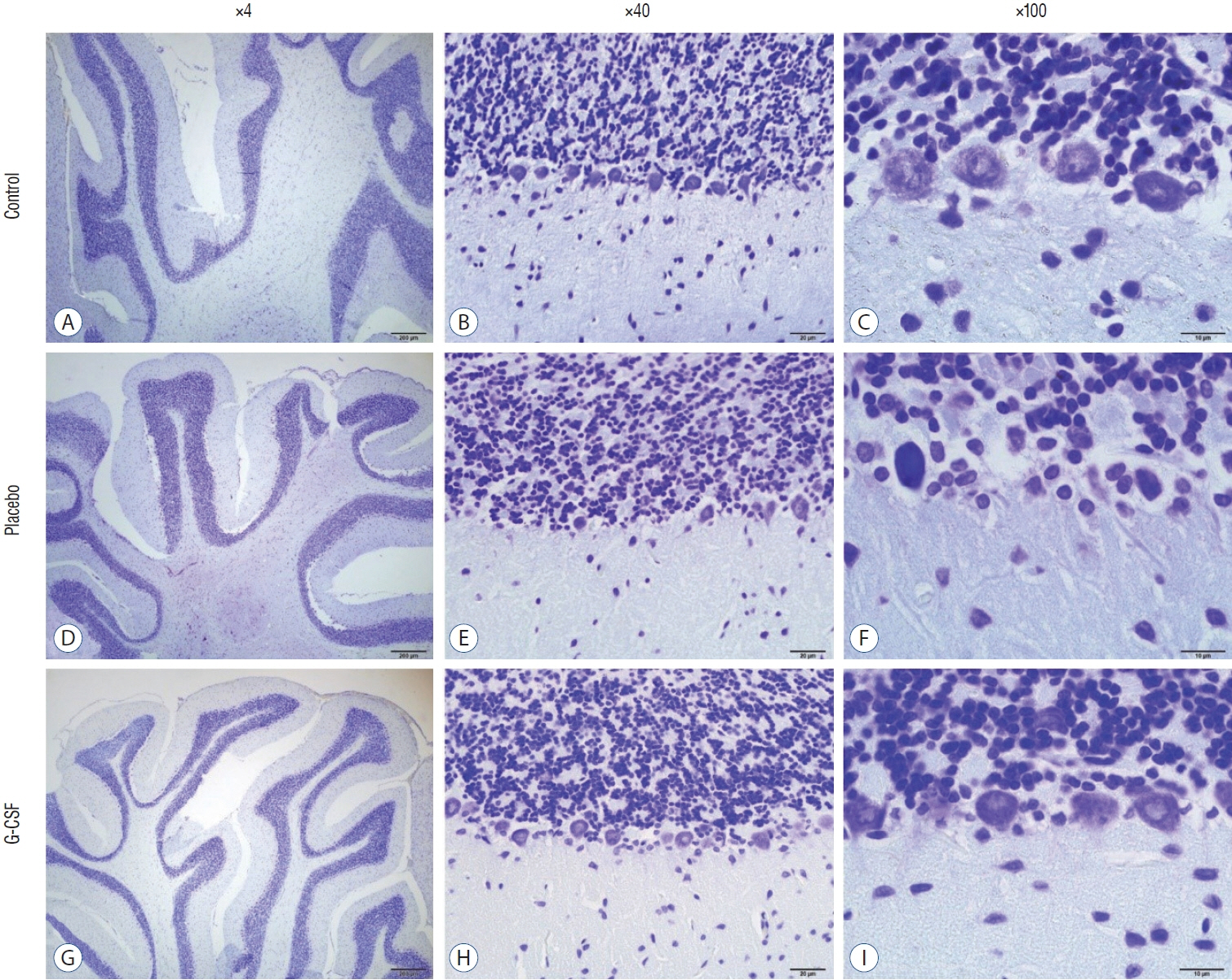
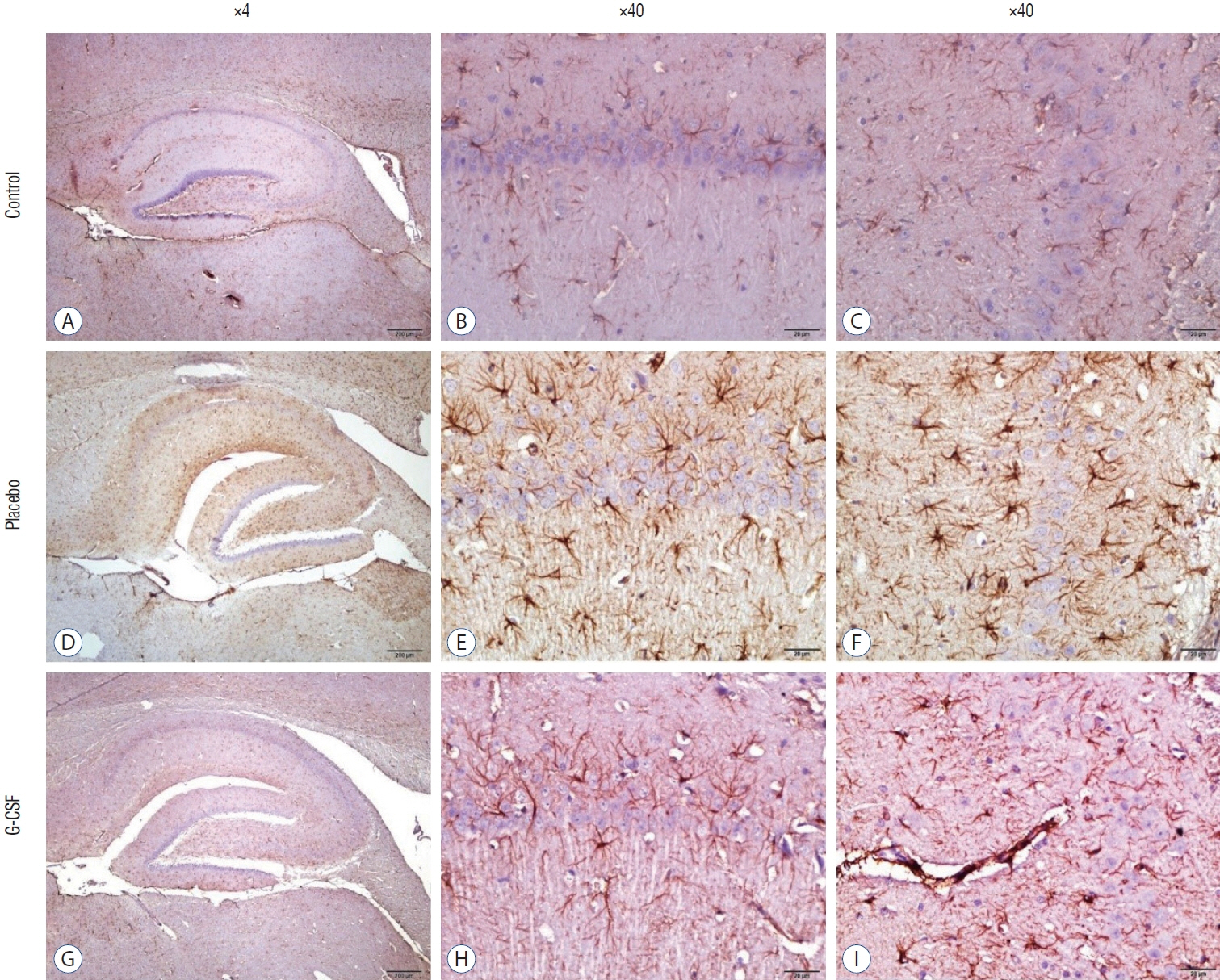
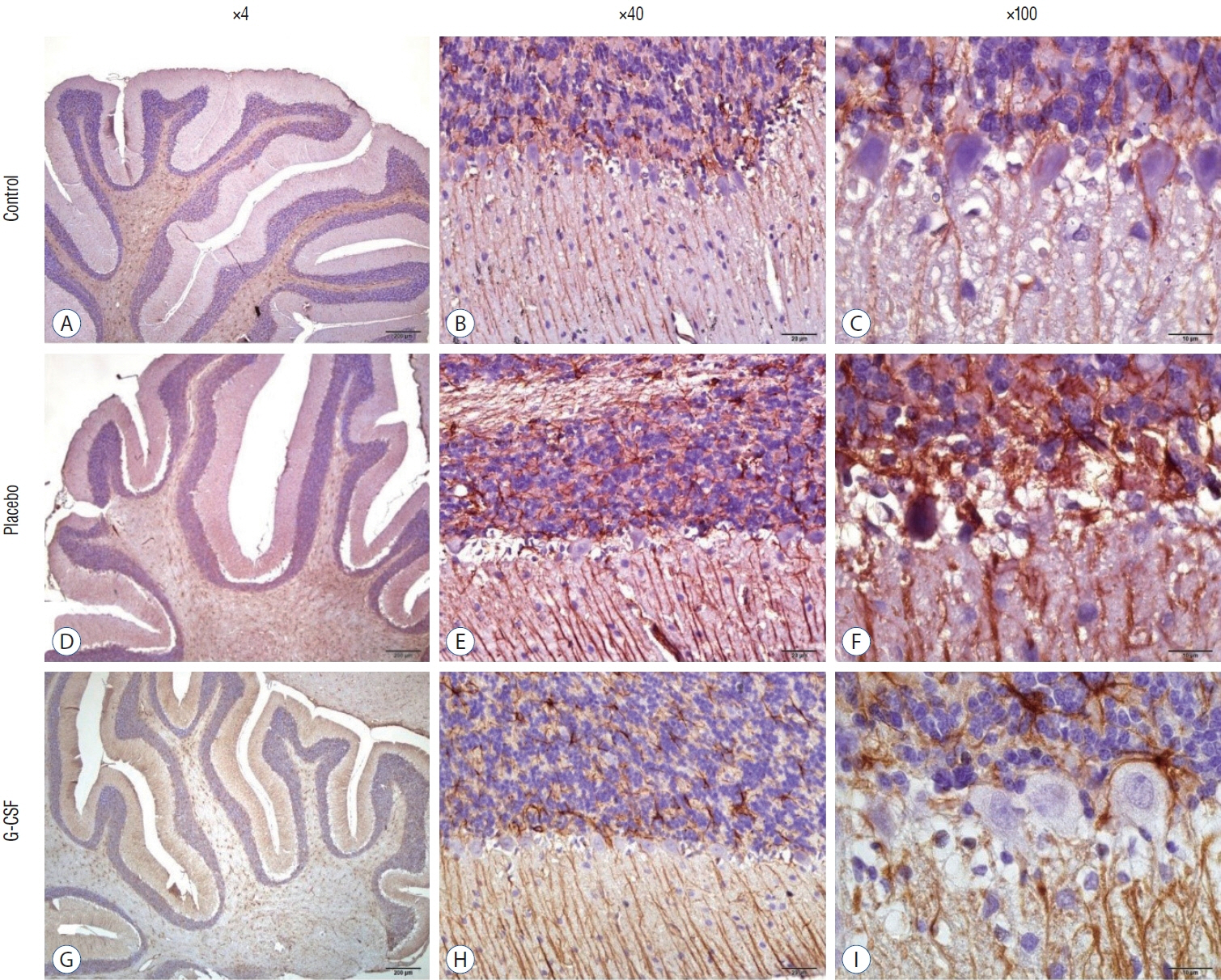
: G-CSF treatment positively affected the results of the three-chamber sociability test, open-space test and passive avoidance learning test, cornu Ammonis (CA) 1, CA3, and Purkinje neuron counts, and the brain levels of brain-derived neurotrophic factor and postsynaptic density protein-95. However, G-CSF treatment reduced the glial fibrillary acidic protein immunostaining index and brain levels of malondialdehyde, tumor necrosis factor-alpha, nuclear factor kappa-B, and lactate. In addition, on MR spectroscopy, G-CSF had a reversible effect on brain lactate levels.
Conclusion
: In this first designed brain irradiation animal model, which evaluated G-CSF effects, we observed that G-CSF had reparative, neuroprotective and anti-neurodegenerative effects and had increased neurotrophic factor expression, neuronal counts, and morphology changes. In addition, G-CSF had a proven lactate-lowering effect in MR spectroscopy and brain materials.
Keyword
Figure
Reference
-
References
1. Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Sanberg PR, SanchezRamos J, et al. Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PLoS One. 9:e90953. 2014.2. Albensi BC. What is nuclear factor kappa B (NF-κB) doing in and to the mitochondrion? Front Cell Dev Biol. 7:154. 2019.3. Bajwa NM, Halavi S, Hamer M, Semple BD, Noble-Haeusslein LJ, Baghchechi M, et al. Mild concussion, but not moderate traumatic brain injury, is associated with long-term depression-like phenotype in mice. PLoS One. 11:e0146886. 2016.4. Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 11:1164–1178. 2015.5. Broussard JI, Redell JB, Zhao J, Maynard ME, Kobori N, Perez A, et al. Mild traumatic brain injury decreases spatial information content and reduces place field stability of hippocampal CA1 neurons. J Neurotrauma. 37:227–235. 2020.6. Carpenter KL, Jalloh I, Hutchinson PJ. Glycolysis and the significance of lactate in traumatic brain injury. Front Neurosci. 9:112. 2015.7. Dela Peña I, Sanberg PR, Acosta S, Tajiri N, Lin SZ, Borlongan CV. Stem cells and G-CSF for treating neuroinflammation in traumatic brain injury: aging as a comorbidity factor. J Neurosurg Sci. 58:145–149. 2014.8. Dumbuya JS, Chen L, Wu JY, Wang B. The role of G-CSF neuroprotective effects in neonatal hypoxic-ischemic encephalopathy (HIE): current status. J Neuroinflammation. 18:55. 2021.9. Eakin K, Miller JP. Mild traumatic brain injury is associated with impaired hippocampal spatiotemporal representation in the absence of histological changes. J Neurotrauma. 29:1180–1187. 2012.10. Erbas O, Erdogan MA, Khalilnezhad A, Gürkan FT, Yiğittürk G, Meral A, et al. Neurobehavioral effects of long-term maternal fructose intake in rat offspring. Int J Dev Neurosci. 69:68–79. 2018.11. Goshen INBAL, Yirmiya R. The role of pro-inflammatory cytokines in memory processes and neural plasticity. Psychoneuroimmunology. 4:337–378. 2007.12. Horáková L, Ondrejicková O, Bachratá K, Vajdová M. Preventive effect of several antioxidants after oxidative stress on rat brain homogenates. Gen Physiol Biophys. 19:195–206. 2000.13. İzci Y, Erbaş YC. Hipokampus: yapısı ve fonksiyonları. Türk Nöroşir Derg. 25:287–295. 2015.14. Kerman M, Cirak B, Ozguner MF, Dagtekin A, Sutcu R, Altuntas I, et al. Does melatonin protect or treat brain damage from traumatic oxidative stress? Exp Brain Res. 163:406–410. 2005.15. Li H, Wang Y. G-CSF improves CUMS-induced depressive behaviors through downregulating Ras/ERK/MAPK signaling pathway. Biochem Biophys Res Commun. 479:827–832. 2016.16. Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, et al. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 25:1481–1488. 1994.17. Meral R. Radyasyonun bilissel fonksiyonlara etkisi. Türk Nörosirürji Dergisi. 17:139–148. 2007.18. Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 191:118–129. 2008.19. Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 3:303–314. 2004.20. Nolan A, Hennessy E, Krukowski K, Guglielmetti C, Chaumeil MM, Sohal VS, et al. Repeated mild head injury leads to wide-ranging deficits in higher-order cognitive functions associated with the prefrontal cortex. J Neurotrauma. 35:2425–2434. 2018.21. Paolin A, Nardin L, Gaetani P, Rodriguez Y Baena R, Pansarasa O, Marzatico F. Oxidative damage after severe head injury and its relationship to neurological outcome. Neurosurgery. 51:949–954. discussion 954-955. 2002.22. Park CH, Joa KL, Lee MO, Yoon SH, Kim MO. The combined effect of granulocyte-colony stimulating factor (G-CSF) treatment and exercise in rats with spinal cord injury. J Spinal Cord Med. 43:339–346. 2020.23. Pendergrass JC, Targum SD, Harrison JE. Cognitive impairment associated with cancer: a brief review. Innov Clin Neurosci. 15:36–44. 2018.24. Rahman R, Sulman E, Haas-Kogan D, Cagney DN. Update on radiation therapy for central nervous system tumors. Hematol Oncol Clin North Am. 36:77–93. 2022.25. Rowland LM, Pradhan S, Korenic S, Wijtenburg SA, Hong LE, Edden RA, et al. Elevated brain lactate in schizophrenia: a 7T magnetic resonance spectroscopy study. Transl Psychiatry. 6:e967. 2016.26. Salberg S, Yamakawa G, Christensen J, Kolb B, Mychasiuk R. Assessment of a nutritional supplement containing resveratrol, prebiotic fiber, and omega-3 fatty acids for the prevention and treatment of mild traumatic brain injury in rats. Neuroscience. 365:146–157. 2017.27. Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 115:2083–2098. 2005.28. Scorza CA, Marques MJG, Gomes da Silva S, Naffah-Mazzacoratti MDG, Scorza FA, Cavalheiro EA. Status epilepticus does not induce acute brain inflammatory response in the Amazon rodent Proechimys, an animal model resistant to epileptogenesis. Neurosci Lett. 668:169–173. 2018.29. Shibamoto Y. Radiation therapy for primary central nervous system lymphoma. Oncol Rev. 7:e4. 2013.30. Shih RH, Wang CY, Yang CM. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci. 8:77. 2015.31. Shyu WC, Lin SZ, Lee CC, Liu DD, Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ. 174:927–933. 2006.32. Sikoglu EM, Heffernan ME, Tam K, Sicard KM, Bratane BT, Quan M, et al. Enhancement in cognitive function recovery by granulocyte-colony stimulating factor in a rodent model of traumatic brain injury. Behav Brain Res. 259:354–356. 2014.33. Solaroglu I, Cahill J, Jadhav V, Zhang JH. A novel neuroprotectant granulocyte-colony stimulating factor. Stroke. 37:1123–1128. 2006.34. Solmaz V, Erdoğan MA, Alnak A, Meral A, Erbaş O. Erythropoietin shows gender dependent positive effects on social deficits, learning/memory impairments, neuronal loss and neuroinflammation in the lipopolysaccharide induced rat model of autism. Neuropeptides. 83:102073. 2020.35. Song S, Kong X, Acosta S, Sava V, Borlongan C, Sanchez-Ramos J. Granulocyte colony-stimulating factor promotes behavioral recovery in a mouse model of traumatic brain injury. J Neurosci Res. 94:409–423. 2016.36. Tan XL, Wright DK, Liu S, Hovens C, O’Brien TJ, Shultz SR. Sodium selenate, a protein phosphatase 2A activator, mitigates hyperphosphorylated tau and improves repeated mild traumatic brain injury outcomes. Neuropharmacology. 108:382–393. 2016.37. Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 3:159–168. 2004.38. Warner DS, Sheng H, Batinić-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 207(Pt 18):3221–3231. 2004.39. Yağmur EN, Yıldız N, Adıgüzel S, Femir B, Şenyer S, Şen M, et al. The role of NF-κb in neuronal plasticity and neurodegenerative diseases. Deneysel Tıp Araştırma Enstitüsü Dergisi. 7:71–85. 2017.40. Yang DY, Chen YJ, Wang MF, Pan HC, Chen SY, Cheng FC. Granulocyte colony-stimulating factor enhances cellular proliferation and motor function recovery on rats subjected to traumatic brain injury. Neurol Res. 32:1041–1049. 2010.41. Yang Z, Wang KK. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 38:364–374. 2015.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sweet Syndrome in a Child with Aplastic Anemia after Receiving Recombinant Granulocyte Colony-stimulating Factor
- Two cases of congenital agranulocytosis treated with recombinant human granulocyte colony-stimulating factor
- The effect of granulocyte colony-stimulating factor in chemotherapy of acute myelogenous leukemia
- The effects on the production of platelet activating factor in the cultured human endothelial cells by interleukin-6 and granulocyte macrophage colony stimulating factor
- Effect of combination gene therapy with herpes simplex virus thymidine kinase suicidal gene and granulocyte-macrophage colony-stimulating factor gene in murine melanoma model