Nutr Res Pract.
2023 Aug;17(4):616-630. 10.4162/nrp.2023.17.4.616.
IPA and its precursors differently modulate the proliferation, differentiation, and integrity of intestinal epithelial cells
- Affiliations
-
- 1Nutrição e Metabolismo, NOVA Medical School - Faculdade de Ciências Médicas (NMS - FCM), Universidade NOVA de Lisboa, 1169-056 Lisboa, Portugal
- 2CINTESIS, NOVA Medical School - Faculdade de Ciências Médicas (NMS - FCM), Universidade NOVA de Lisboa, 1169-056 Lisboa, Portugal
- 3CHRC, NOVA Medical School - Faculdade de Ciências Médicas (NMS - FCM), Universidade NOVA de Lisboa, 1169-056 Lisboa, Portugal
- 4Unidade Universitária Lifestyle Medicine José de Mello Saúde by NOVA Medical School, 1169-056 Lisboa, Portugal
- KMID: 2545180
- DOI: http://doi.org/10.4162/nrp.2023.17.4.616
Abstract
- BACKGROUND/OBJECTIVES
Indole-3-propionic acid (IPA) is a tryptophan-derived microbial metabolite that has been associated with protective effects against inflammatory and metabolic diseases. However, there is a lack of knowledge regarding the effects of IPA under physiological conditions and at the intestinal level.
MATERIALS/METHODS
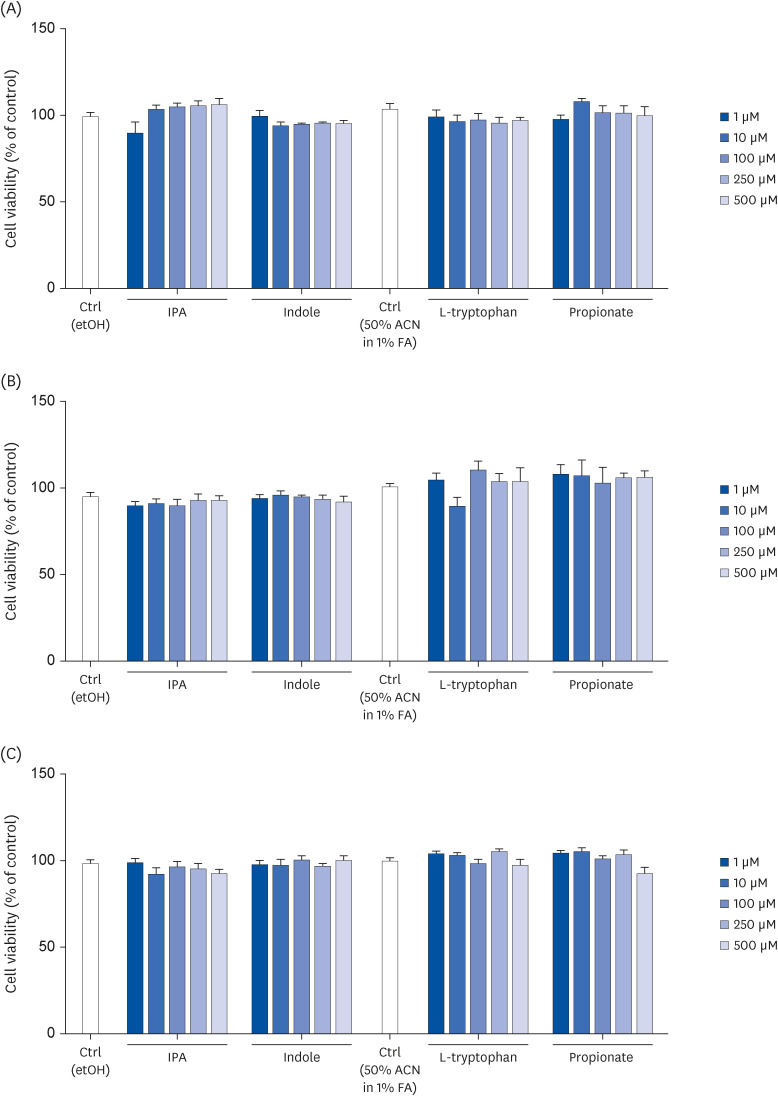
Human intestinal epithelial Caco-2 cells were treated for 2, 24, and/ or 72 h with IPA or its precursors – indole, tryptophan, and propionate – at 1, 10, 100, 250, or 500 μM to assess cell viability, integrity, differentiation, and proliferation.
RESULTS
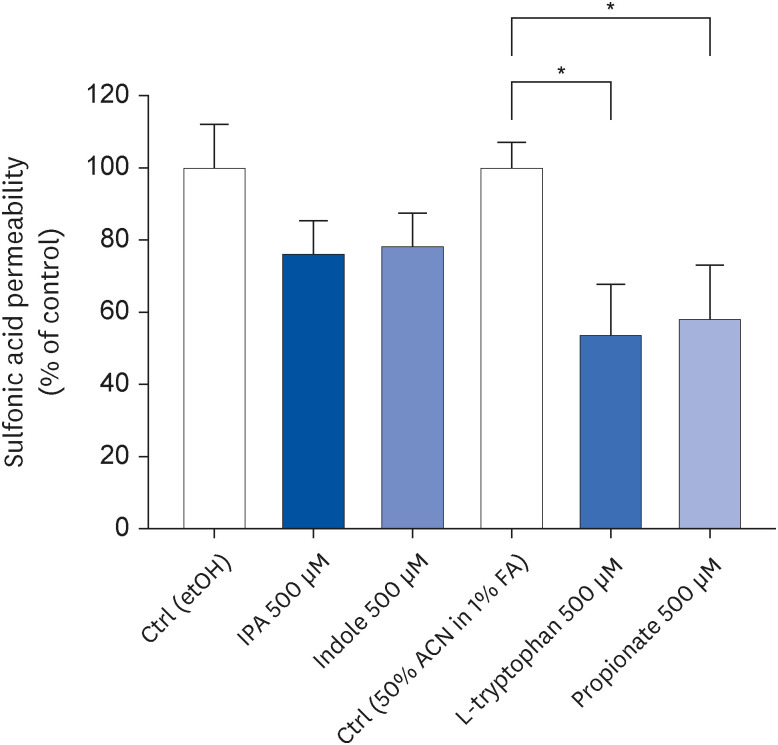
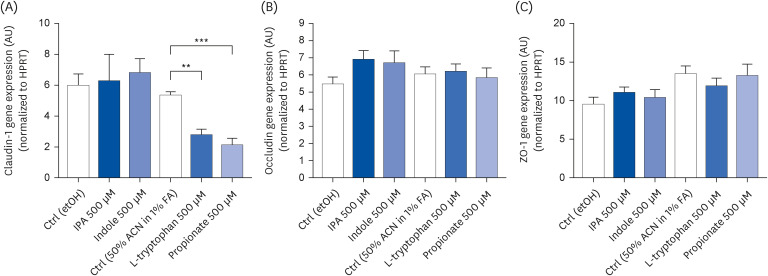
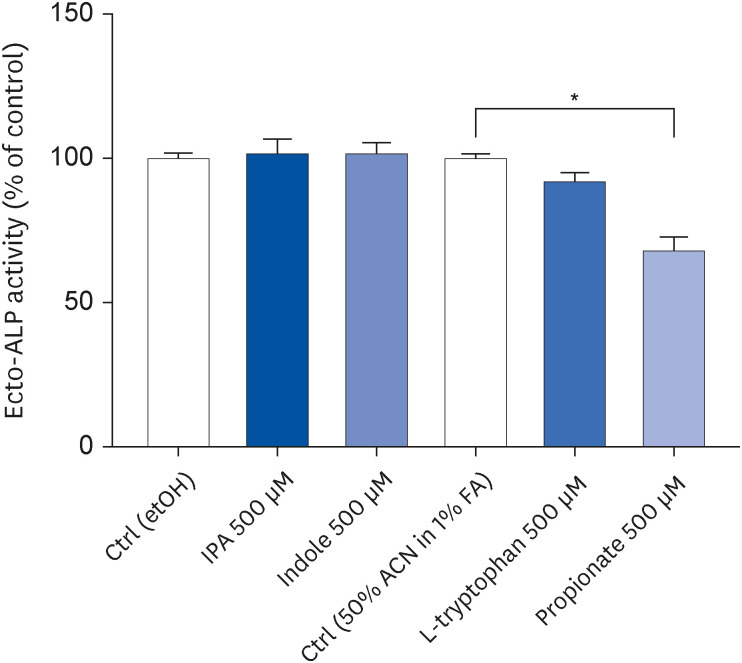
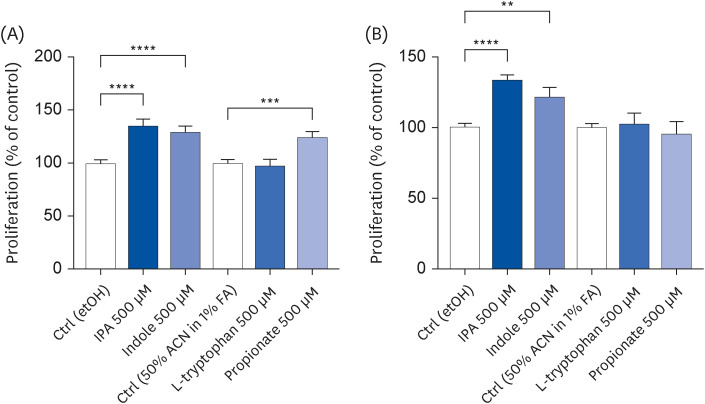
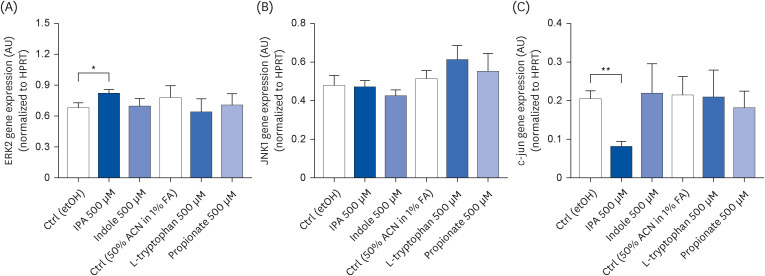
IPA induced cell proliferation and this effect was associated with a higher expression of extracellular signal-regulated kinase 2 (ERK2) and a lower expression of c-Jun. Although indole and propionate also induced cell proliferation, this involved ERK2 and c-Jun independent mechanisms. On the other hand, both tryptophan and propionate increased cell integrity and reduced the expression of claudin-1, whereas propionate decreased cell differentiation.
CONCLUSIONS
In conclusion, these findings suggested that IPA and its precursors distinctly contribute to the proliferation, differentiation, and barrier function properties of human intestinal epithelial cells. Moreover, the pro-proliferative effect of IPA in intestinal epithelial cells was not explained by its precursors and is rather related to its whole chemical structure. Maintaining IPA at physiological levels, e.g., through IPA-producing commensal bacteria, may be important to preserve the integrity of the intestinal barrier and play an integral role in maintaining metabolic homeostasis.
Keyword
Figure
Reference
-
1. Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019; 7:91. PMID: 31196177.
Article2. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014; 14:141–153. PMID: 24566914.
Article3. Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020; 17:223–237. PMID: 32076145.
Article4. Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021; 70:1174–1182. PMID: 33272977.
Article5. Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018; 57:1–24.
Article6. Neis EP, Dejong CH, Rensen SS. The role of microbial amino acid metabolism in host metabolism. Nutrients. 2015; 7:2930–2946. PMID: 25894657.
Article7. Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018; 9:3294. PMID: 30120222.
Article8. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018; 23:716–724. PMID: 29902437.
Article9. Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A. 2010; 107:228–233. PMID: 19966295.
Article10. Sun M, Ma N, He T, Johnston LJ, Ma X. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit Rev Food Sci Nutr. 2020; 60:1760–1768. PMID: 30924357.
Article11. Jennis M, Cavanaugh CR, Leo GC, Mabus JR, Lenhard J, Hornby PJ. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo . Neurogastroenterol Motil. 2018; 30:e13178.
Article12. Hyland NP, Cavanaugh CR, Hornby PJ. Emerging effects of tryptophan pathway metabolites and intestinal microbiota on metabolism and intestinal function. Amino Acids. 2022; 54:57–70. PMID: 35038025.
Article13. Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017; 551:648–652. PMID: 29168502.
Article14. Alexeev EE, Lanis JM, Kao DJ, Campbell EL, Kelly CJ, Battista KD, Gerich ME, Jenkins BR, Walk ST, Kominsky DJ, et al. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am J Pathol. 2018; 188:1183–1194. PMID: 29454749.
Article15. de Mello VD, Paananen J, Lindström J, Lankinen MA, Shi L, Kuusisto J, Pihlajamäki J, Auriola S, Lehtonen M, Rolandsson O, et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep. 2017; 7:46337. PMID: 28397877.
Article16. Tuomainen M, Lindström J, Lehtonen M, Auriola S, Pihlajamäki J, Peltonen M, Tuomilehto J, Uusitupa M, de Mello VD, Hanhineva K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr Diabetes. 2018; 8:35. PMID: 29795366.
Article17. Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014; 41:296–310. PMID: 25065623.
Article18. Garg A, Zhao A, Erickson SL, Mukherjee S, Lau AJ, Alston L, Chang TK, Mani S, Hirota SA. Pregnane X receptor activation attenuates inflammation-associated intestinal epithelial barrier dysfunction by inhibiting cytokine-induced myosin light-chain kinase expression and c-Jun N-terminal kinase 1/2 activation. J Pharmacol Exp Ther. 2016; 359:91–101. PMID: 27440420.
Article19. Konopelski P, Konop M, Gawrys-Kopczynska M, Podsadni P, Szczepanska A, Ufnal M. Indole-3-propionic acid, a tryptophan-derived bacterial metabolite, reduces weight gain in rats. Nutrients. 2019; 11:591. PMID: 30862081.
Article20. Abildgaard A, Elfving B, Hokland M, Wegener G, Lund S. The microbial metabolite indole-3-propionic acid improves glucose metabolism in rats, but does not affect behaviour. Arch Physiol Biochem. 2018; 124:306–312. PMID: 29113509.
Article21. Valenzano MC, DiGuilio K, Mercado J, Teter M, To J, Ferraro B, Mixson B, Manley I, Baker V, Moore BA, et al. Remodeling of tight junctions and enhancement of barrier integrity of the CACO-2 intestinal epithelial cell layer by micronutrients. PLoS One. 2015; 10:e0133926. PMID: 26226276.
Article22. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63. PMID: 6606682.
Article23. Herminghaus A, Eberhardt R, Truse R, Schulz J, Bauer I, Picker O, Vollmer C. Nitroglycerin and iloprost improve mitochondrial function in colon homogenate without altering the barrier integrity of Caco-2 monolayers. Front Med (Lausanne). 2018; 5:291. PMID: 30460235.
Article24. Shin J, Carr A, Corner GA, Tögel L, Dávalos-Salas M, Tran H, Chueh AC, Al-Obaidi S, Chionh F, Ahmed N, et al. The intestinal epithelial cell differentiation marker intestinal alkaline phosphatase (ALPi) is selectively induced by histone deacetylase inhibitors (HDACi) in colon cancer cells in a Kruppel-like factor 5 (KLF5)-dependent manner. J Biol Chem. 2014; 289:25306–25316. PMID: 25037223.
Article25. Calhau C, Martel F, Hipólito-Reis C, Azevedo I. Effect of thiamine on 3H-MPP+ uptake by Caco-2 cells. Pharmacol Res. 2003; 48:579–584. PMID: 14527822.
Article26. Gonçalves P, Gregório I, Catarino TA, Martel F. The effect of oxidative stress upon the intestinal epithelial uptake of butyrate. Eur J Pharmacol. 2013; 699:88–100. PMID: 23201076.
Article27. Klingbeil E, de La Serre CB. Microbiota modulation by eating patterns and diet composition: impact on food intake. Am J Physiol Regul Integr Comp Physiol. 2018; 315:R1254–R1260. PMID: 30230934.
Article28. Lin R, Liu W, Piao M, Zhu H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids. 2017; 49:2083–2090. PMID: 28932911.
Article29. Rodríguez-Romero JJ, Durán-Castañeda AC, Cárdenas-Castro AP, Sánchez-Burgos JA, Zamora-Gasga VM, Sáyago-Ayerdi SG. What we know about protein gut metabolites: Implications and insights for human health and diseases. Food Chem X. 2021; 13:100195. PMID: 35499004.
Article30. Cussotto S, Delgado I, Anesi A, Dexpert S, Aubert A, Beau C, Forestier D, Ledaguenel P, Magne E, Mattivi F, et al. Tryptophan metabolic pathways are altered in obesity and are associated with systemic inflammation. Front Immunol. 2020; 11:557. PMID: 32351500.
Article31. Zhao ZH, Xin FZ, Xue Y, Hu Z, Han Y, Ma F, Zhou D, Liu XL, Cui A, Liu Z, et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp Mol Med. 2019; 51:1–14.
Article32. Lee DM, Ecton KE, Trikha SR, Wrigley SD, Thomas KN, Battson ML, Wei Y, Johnson SA, Weir TL, Gentile CL. Microbial metabolite indole-3-propionic acid supplementation does not protect mice from the cardiometabolic consequences of a Western diet. Am J Physiol Gastrointest Liver Physiol. 2020; 319:G51–G62. PMID: 32421360.
Article33. Kaufmann SH. Indole propionic acid: a small molecule links between gut microbiota and tuberculosis. Antimicrob Agents Chemother. 2018; 62:e00389-18. PMID: 29700005.
Article34. Verhoeckx K, Cotter P, López-Expósito I, Kleiveland C, Lea T, Mackie A, et al. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models. Heidelberg: Springer International Publishing;2015.35. Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle. 2009; 8:1168–1175. PMID: 19282669.
Article36. Mechta-Grigoriou F, Gerald D, Yaniv M. The mammalian Jun proteins: redundancy and specificity. Oncogene. 2001; 20:2378–2389. PMID: 11402334.
Article37. Yin J, Sheng B, Qiu Y, Yang K, Xiao W, Yang H. Role of AhR in positive regulation of cell proliferation and survival. Cell Prolif. 2016; 49:554–560. PMID: 27523394.
Article38. Krautkramer KA, Fan J, Bäckhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol. 2021; 19:77–94. PMID: 32968241.
Article39. Li J, Zhang L, Wu T, Li Y, Zhou X, Ruan Z. Indole-3-propionic acid improved the intestinal barrier by enhancing epithelial barrier and mucus barrier. J Agric Food Chem. 2021; 69:1487–1495. PMID: 33356219.
Article40. Poormasjedi-Meibod MS, Jalili RB, Hosseini-Tabatabaei A, Hartwell R, Ghahary A. Immuno-regulatory function of indoleamine 2,3 dioxygenase through modulation of innate immune responses. PLoS One. 2013; 8:e71044. PMID: 23940687.
Article41. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002; 196:459–468. PMID: 12186838.
Article42. Pham HT, Ono M, Hara ES, Nguyen HT, Dang AT, Do HT, Komori T, Tosa I, Hazehara-Kunitomo Y, Yoshioka Y, et al. Tryptophan and kynurenine enhances the stemness and osteogenic differentiation of bone marrow-derived mesenchymal stromal cells in vitro and in vivo . Materials (Basel). 2021; 14:208. PMID: 33406724.
Article43. Bindels LB, Porporato P, Dewulf EM, Verrax J, Neyrinck AM, Martin JC, Scott KP, Buc Calderon P, Feron O, Muccioli GG, et al. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br J Cancer. 2012; 107:1337–1344. PMID: 22976799.
Article44. Venegas DP, De Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs) -mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019; 10:277. PMID: 30915065.45. Fu H, Shi YQ, Mo SJ. Effect of short-chain fatty acids on the proliferation and differentiation of the human colonic adenocarcinoma cell line Caco-2. Chin J Dig Dis. 2004; 5:115–117. PMID: 15612246.
Article46. Park BO, Kim SH, Kim JH, Kim SY, Park BC, Han SB, Park SG, Kim JH, Kim S. The short-chain fatty acid receptor GPR43 modulates YAP/TAZ via RhoA. Mol Cells. 2021; 44:458–467. PMID: 34112743.
Article47. Koopmans SJ, Guzik AC, van der Meulen J, Dekker R, Kogut J, Kerr BJ, Southern LL. Effects of supplemental L-tryptophan on serotonin, cortisol, intestinal integrity, and behavior in weanling piglets. J Anim Sci. 2006; 84:963–971. PMID: 16543575.
Article48. Chen M, Liu Y, Xiong S, Wu M, Li B, Ruan Z, Hu X. Dietary l-tryptophan alleviated LPS-induced intestinal barrier injury by regulating tight junctions in a Caco-2 cell monolayer model. Food Funct. 2019; 10:2390–2398. PMID: 30977499.
Article49. Feng Y, Wang Y, Wang P, Huang Y, Wang F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell Physiol Biochem. 2018; 49:190–205. PMID: 30138914.
Article50. Adesina PA, Isayama K, Sitolo GC, Yamamoto Y, Suzuki T. Propionate and dietary fermentable fibers upregulate intestinal heat shock protein70 in intestinal Caco-2 cells and mouse colon. J Agric Food Chem. 2021; 69:8460–8470. PMID: 34291640.
Article51. van der Hee B, Wells JM. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021; 29:700–712. PMID: 33674141.
Article52. Pope JL, Ahmad R, Bhat AA, Washington MK, Singh AB, Dhawan P. Claudin-1 overexpression in intestinal epithelial cells enhances susceptibility to adenamatous polyposis coli-mediated colon tumorigenesis. Mol Cancer. 2014; 13:167. PMID: 24997475.
Article53. Garcia-Hernandez V, Quiros M, Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann N Y Acad Sci. 2017; 1397:66–79. PMID: 28493289.
Article54. Shen Z, Song W, Qian L, Zhu J, Li Y, Li M, Zhang T, Zhao W, Zhou Y, Yang X. Effect of claudin 1 on cell proliferation, migration and apoptosis in human cervical squamous cell carcinoma. Oncol Rep. 2021; 45:606–618. PMID: 33416141.
Article55. Filippone A, Lanza M, Campolo M, Casili G, Paterniti I, Cuzzocrea S, Esposito E. The anti-inflammatory and antioxidant effects of sodium propionate. Int J Mol Sci. 2020; 21:3026. PMID: 32344758.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Studies on Functional Differentiation of Small Intestinal Epithelial Cells
- Effect of endothelin-1 on proliferation and differentiation of rat tracheal epithelial cells
- Host-Microbe Interactions Regulate Intestinal Stem Cells and Tissue Turnover in Drosophila
- Role of DNA Methylation in the Development and Differentiation of Intestinal Epithelial Cells and Smooth Muscle Cells
- Roles of intestinal epithelial cells in the maintenance of gut homeostasis