Endocrinol Metab.
2023 Jun;38(3):338-346. 10.3803/EnM.2023.1664.
The Early Changes in Thyroid-Stimulating Immunoglobulin Bioassay over Anti-Thyroid Drug Treatment Could Predict Prognosis of Graves’ Disease
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2543320
- DOI: http://doi.org/10.3803/EnM.2023.1664
Abstract
- Background
To determine whether baseline thyroid-stimulating immunoglobulin (TSI) bioassay or its early response upon treatment with an anti-thyroid drug (ATD) can predict prognosis of Graves’ disease (GD) in real-world practice.
Methods
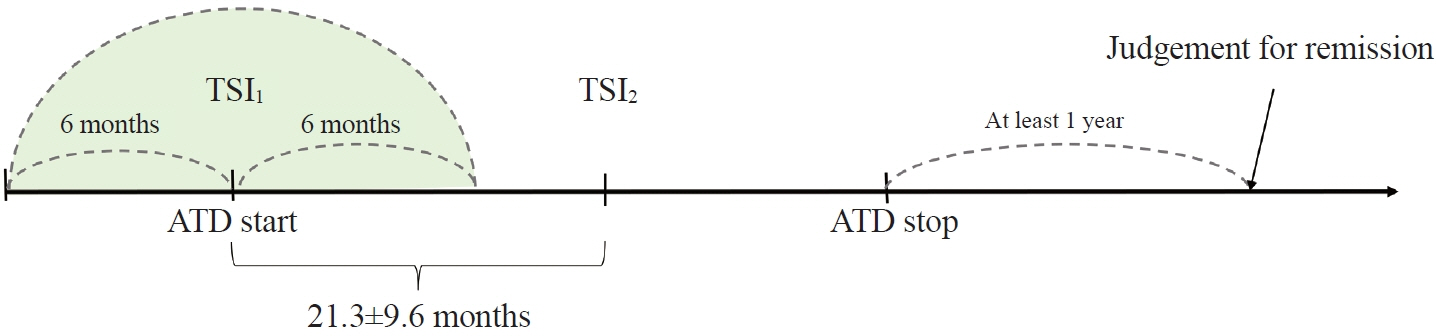
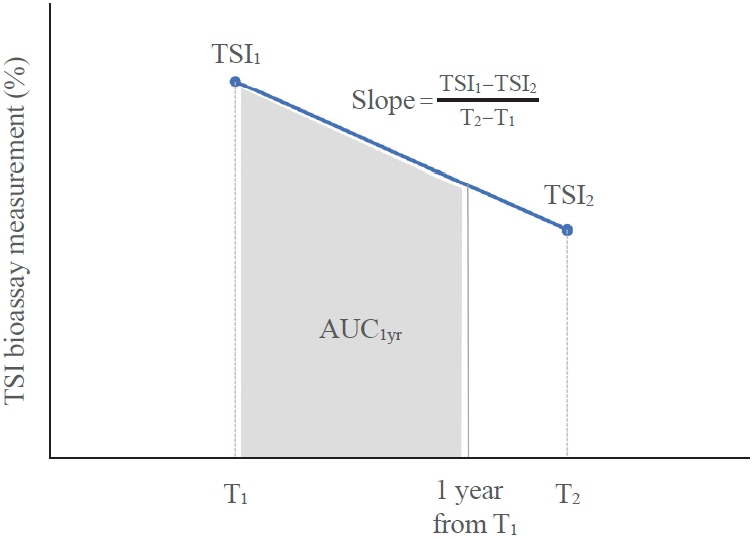
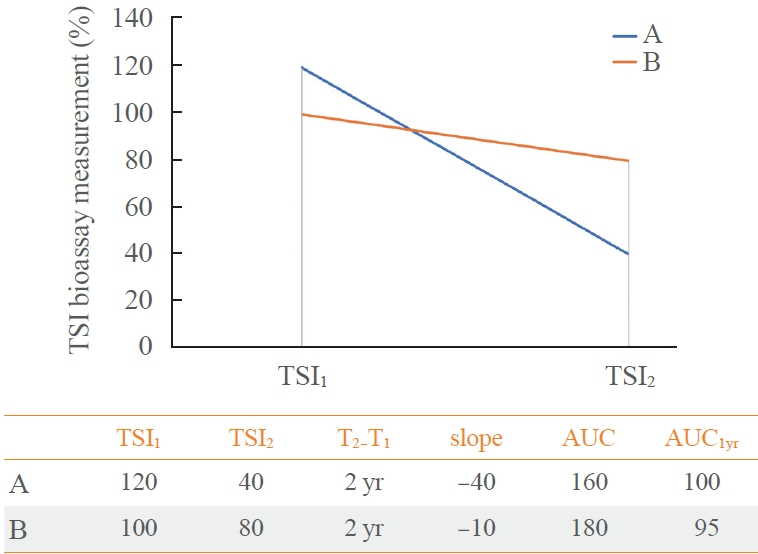
This retrospective study enrolled GD patients who had previous ATD treatment with TSI bioassay checked at baseline and at follow-up from April 2010 to November 2019 in one referral hospital. The study population were divided into two groups: patients who experienced relapse or continued ATD (relapse/persistence), and patients who experienced no relapse after ATD discontinuation (remission). The slope and area under the curve at 1st year (AUC1yr) of thyroid-stimulating hormone receptor antibodies including TSI bioassay and thyrotropin-binding inhibitory immunoglobulin (TBII) were calculated as differences between baseline and second values divided by time duration (year).
Results
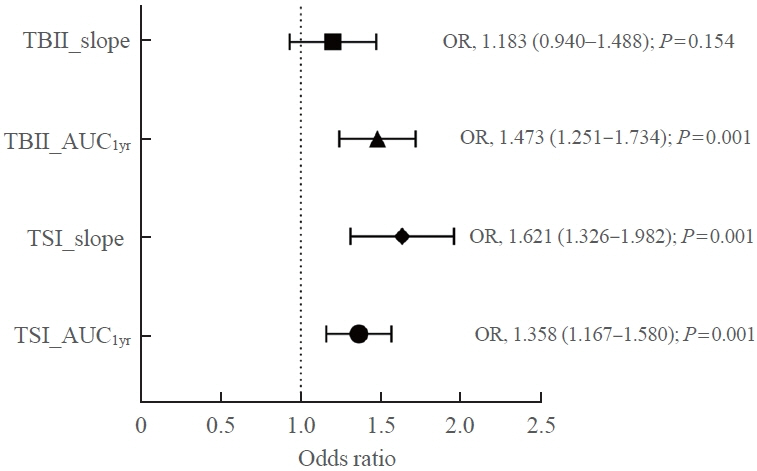
Among enrolled 156 study subjects, 74 (47.4%) had relapse/persistence. Baseline TSI bioassay values did not show significant differences between the two groups. However, the relapse/persistence group showed less decremental TSI bioassay in response to ATD than the remission group (–84.7 [TSI slope, –198.2 to 8.2] vs. –120.1 [TSI slope, –204.4 to –45.9], P=0.026), whereas the TBII slope was not significantly different between the two groups. The relapse/persistence group showed higher AUC1yr of TSI bioassay and TBII in the 1st year during ATD treatment than the remission group (AUC1yr for TSI bioassay, P=0.0125; AUC1yr for TBII,P =0.001).
Conclusion
Early changes in TSI bioassay can better predict prognosis of GD than TBII. Measurement of TSI bioassay at beginning and follow-up could help predict GD prognosis.
Keyword
Figure
Cited by 1 articles
-
Dynamic Risk Model for the Medical Treatment of Graves’ Hyperthyroidism according to Treatment Duration
Meihua Jin, Chae A Kim, Min Ji Jeon, Won Bae Kim, Tae Yong Kim, Won Gu Kim
Endocrinol Metab. 2024;39(4):579-589. doi: 10.3803/EnM.2024.1918.
Reference
-
1. Kahaly GJ. Management of Graves thyroidal and extrathyroidal disease: an update. J Clin Endocrinol Metab. 2020; 105:3704–20.
Article2. Smith TJ, Hegedus L. Graves’ disease. N Engl J Med. 2016; 375:1552–65.
Article3. Baek HS, Park JY, Jeong CH, Ha J, Kang MI, Lim DJ. Usefulness of real-time quantitative microvascular ultrasonography for differentiation of Graves’ disease from destructive thyroiditis in thyrotoxic patients. Endocrinol Metab (Seoul). 2022; 37:323–32.
Article4. Kahaly GJ, Diana T, Kanitz M, Frommer L, Olivo PD. Prospective trial of functional thyrotropin receptor antibodies in Graves disease. J Clin Endocrinol Metab. 2020; 105:e1006–14.
Article5. Giuliani C, Saji M, Bucci I, Napolitano G. Bioassays for TSH receptor autoantibodies, from FRTL-5 cells to TSH receptor-LH/CG receptor chimeras: the contribution of Leonard D. Kohn. Front Endocrinol (Lausanne). 2016; 7:103.
Article6. Alvin Mathew A, Papaly R, Maliakal A, Chandra L, Antony MA. Elevated Graves’ disease-specific thyroid-stimulating immunoglobulin and thyro tibody as a predictive factor for Graves’ disease relapse. Cureus. 2022; 14:e22190.7. Liu X, Wong CK, Chan WW, Tang EH, Woo YC, Lam CL, et al. Outcomes of Graves’ disease patients following antithyroid drugs, radioactive iodine, or thyroidectomy as the first-line treatment. Ann Surg. 2021; 273:1197–206.
Article8. Kim MJ, Cho SW, Kim YA, Choi HS, Park YJ, Park DJ, et al. Clinical outcomes of repeated radioactive iodine therapy for Graves’ disease. Endocrinol Metab (Seoul). 2022; 37:524–32.
Article9. Da Silva Santos T, Oliveira JC, Freitas C, Couto de Carvalho A. Thyroid-stimulatory antibody as a predictive factor for Graves’ disease relapse. Cureus. 2022; 14:e22190.
Article10. Barbesino G, Tomer Y. Clinical review: clinical utility of TSH receptor antibodies. J Clin Endocrinol Metab. 2013; 98:2247–55.11. Bartalena L, Piantanida E, Gallo D, Ippolito S, Tanda ML. Management of Graves’ hyperthyroidism: present and future. Expert Rev Endocrinol Metab. 2022; 17:153–66.
Article12. Brito JP, Payne S, Singh Ospina N, Rodriguez-Gutierrez R, Maraka S, Sangaralingham LR, et al. Patterns of use, efficacy, and safety of treatment options for patients with Graves’ disease: a nationwide population-based study. Thyroid. 2020; 30:357–64.
Article13. Azizi F, Amouzegar A, Tohidi M, Hedayati M, Khalili D, Cheraghi L, et al. Increased remission rates after long-term methimazole therapy in patients with Graves’ disease: results of a randomized clinical trial. Thyroid. 2019; 29:1192–200.
Article14. Park SY, Kim BH, Kim M, Hong AR, Park J, Park H, et al. The longer the antithyroid drug is used, the lower the relapse rate in Graves’ disease: a retrospective multicenter cohort study in Korea. Endocrine. 2021; 74:120–7.
Article15. Azizi F, Abdi H, Mehran L, Amouzegar A. Appropriate duration of antithyroid drug treatment as a predictor for relapse of Graves’ disease: a systematic scoping review. J Endocrinol Invest. 2022; 45:1139–50.
Article16. Garcia-Mayor RV, Alvarez-Vazquez P, Fluiters E, Valverde D, Andrade A. Long-term remission following antithyroid drug withdrawal in patients with Graves’ hyperthyroidism: parameters with prognostic value. Endocrine. 2019; 63:316–22.
Article17. Baek HS, Lee J, Jeong CH, Lee J, Ha J, Jo K, et al. The prediction model using thyroid-stimulating immunoglobulin bioassay for relapse of Graves’ disease. J Endocr Soc. 2022; 6:bvac023.
Article18. Kwon H, Kim WG, Jang EK, Kim M, Park S, Jeon MJ, et al. Usefulness of measuring thyroid stimulating antibody at the time of antithyroid drug withdrawal for predicting relapse of Graves disease. Endocrinol Metab (Seoul). 2016; 31:300–10.
Article19. Li Y, Kim J, Diana T, Klasen R, Olivo PD, Kahaly GJ. A novel bioassay for anti-thyrotrophin receptor autoantibodies detects both thyroid-blocking and stimulating activity. Clin Exp Immunol. 2013; 173:390–7.
Article20. Watson PF, Ajjan RA, Phipps J, Metcalfe R, Weetman AP. A new chemiluminescent assay for the rapid detection of thyroid stimulating antibodies in Graves’ disease. Clin Endocrinol (Oxf). 1998; 49:577–81.
Article21. Okamura K, Bandai S, Fujikawa M, Sato K, Ikenoue H, Kitazono T. Long-term antithyroid drug treatment: trends in serum TSH and TSH receptor antibody changes in patients with Graves’ disease. Int J Endocrinol Metab. 2020; 18(Suppl):e101139.
Article22. Takasu N, Yamashiro K, Komiya I, Ochi Y, Sato Y, Nagata A. Remission of Graves’ hyperthyroidism predicted by smooth decreases of thyroid-stimulating antibody and thyrotropinbinding inhibitor immunoglobulin during antithyroid drug treatment. Thyroid. 2000; 10:891–6.
Article23. Kahaly GJ, Bartalena L, Hegedus L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association guideline for the management of Graves’ hyperthyroidism. Eur Thyroid J. 2018; 7:167–86.
Article24. Kahaly GJ, Diana T, Olivo PD. TSH receptor antibodies: relevance & utility. Endocr Pract. 2020; 26:97–106.
Article25. Morshed SA, Ma R, Latif R, Davies TF. How one TSH receptor antibody induces thyrocyte proliferation while another induces apoptosis. J Autoimmun. 2013; 47:17–24.
Article26. Flynn JC, Gilbert JA, Meroueh C, Snower DP, David CS, Kong YC, et al. Chronic exposure in vivo to thyrotropin receptor stimulating monoclonal antibodies sustains high thyroxine levels and thyroid hyperplasia in thyroid autoimmunity-prone HLA-DRB1*0301 transgenic mice. Immunology. 2007; 122:261–7.
Article27. Frohlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017; 8:521.28. Bano A, Gan E, Addison C, Narayanan K, Weaver JU, Tsatlidis V, et al. Age may influence the impact of TRAbs on thyroid function and relapse-risk in patients with Graves disease. J Clin Endocrinol Metab. 2019; 104:1378–85.
Article29. Zuhur SS, Yildiz I, Altuntas Y, Bayraktaroglu T, Erol S, Sahin S, et al. The effect of gender on response to antithyroid drugs and risk of relapse after discontinuation of the antithyroid drugs in patients with Graves’ hyperthyroidism: a multicentre study. Endokrynol Pol. 2020; 71:207–12.30. Zhou Y, Zhou M, Qi Y, Wang W, Chen X, Wang S. The prognostic value of thyroid-stimulating immunoglobulin in the management of Graves’ disease. Ther Adv Endocrinol Metab. 2021; 12:20420188211044943.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Implication of TSH Receptor Antibody Measurement
- Diagnosis and Treatment of Hyperthyroidism in Pregnancy
- Radioactive Iodine-Induced Graves' Disease in a Patient with Toxic Adenoma
- Simultaneous measurement of thyroid growth stimulating antibody and thyroid adenylate cyclase stimulating antibody using FRTL-5 cells in patients with Graves' disease
- A Case Report of Hyperthyroidism and Hypothyroidism Converted Three Times in a Graves' Disease Patient