Nutr Res Pract.
2023 Jun;17(3):408-420. 10.4162/nrp.2023.17.3.408.
Dopamine and serotonin alterations by Hizikia fusiformis extracts under in vitro cortical primary neuronal cell cultures
- Affiliations
-
- 1Department of Food Science and Nutrition, Daegu Catholic University, Gyeongsan 38430, Korea
- 2Department of Food and Life Sciences, Pukyoung National University, Busan 48513, Korea
- 3Department of Artificial Intelligence and Technology, Graduate School of Informatics, Kyoto University, Kyoto 606-8501, Japan
- KMID: 2542839
- DOI: http://doi.org/10.4162/nrp.2023.17.3.408
Abstract
- BACKGROUND/OBJECTIVES
Hizikia fusiformis (HF) is a class of brown seaweeds whose active ingredients exert central nervous system protective effects, such as neuroprotection; however, the underlying mechanisms remain unknown. Given that dopamine (DA) and serotonin (5HT) are two major neurotransmitters involved in various psychiatric disorders and neuronal growth in early neurodevelopmental processes, we investigated whether HF extract could modulate the molecular expression associated with DA and 5HT transmission as well as the structural formation of neurons.
MATERIALS/METHODS
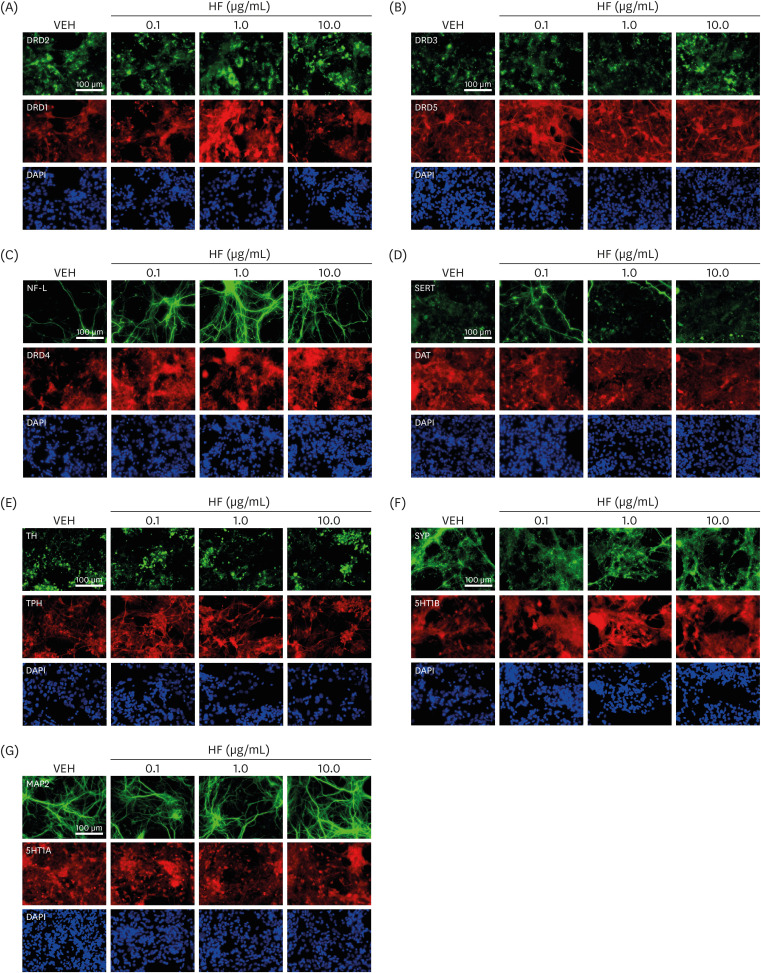
in vitro cell cultures were prepared from cerebral cortical neurons obtained from CD-1 mice on embryonic day 14. Cultured cells were treated with 0.1, 1.0, or 10.0 μg/mL of HT extract for 24 h, followed by fluorescence immunostaining for DA and 5HT-related receptors and transporters and some neuronal structural formation-associated molecules.
RESULTS
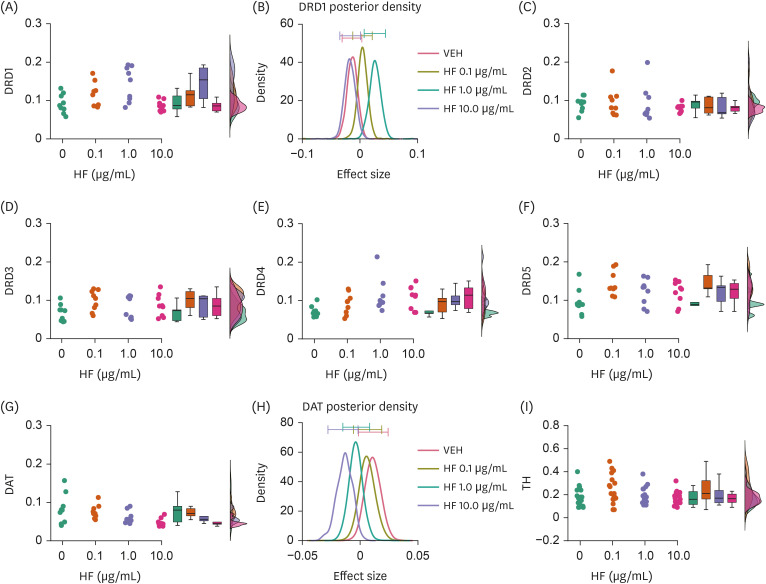
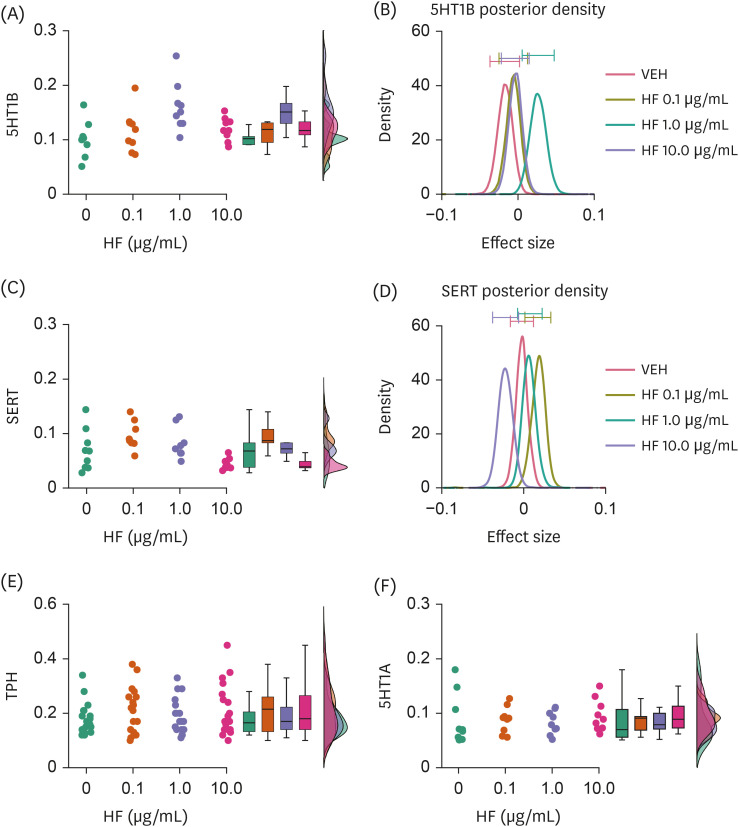
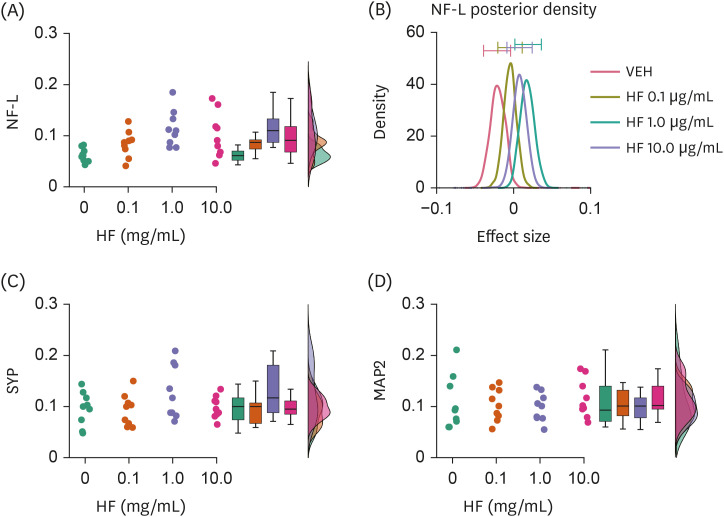
HF extract dose-dependently upregulated the expression levels of selective DA and 5HT receptors, and downregulated the levels of DA and 5HT transporters. Moreover, HF extract increased the neurofilament light chain expression.
CONCLUSION
These results suggest that HF may modulate DA and 5HT transmission, thereby affecting neurodevelopment.
Keyword
Figure
Reference
-
1. Cabana-Domínguez J, Torrico B, Reif A, Fernàndez-Castillo N, Cormand B. Comprehensive exploration of the genetic contribution of the dopaminergic and serotonergic pathways to psychiatric disorders. Transl Psychiatry. 2022; 12:11. PMID: 35013130.2. Lin SH, Lee LT, Yang YK. Serotonin and mental disorders: a concise review on molecular neuroimaging evidence. Clin Psychopharmacol Neurosci. 2014; 12:196–202. PMID: 25598822.3. McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci. 2019; 42:205–220. PMID: 30621912.4. Oades RD. Dopamine-serotonin interactions in attention-deficit hyperactivity disorder (ADHD). Prog Brain Res. 2008; 172:543–565. PMID: 18772050.5. Huang KW, Ochandarena NE, Philson AC, Hyun M, Birnbaum JE, Cicconet M, Sabatini BL. Molecular and anatomical organization of the dorsal raphe nucleus. eLife. 2019; 8:e46464. PMID: 31411560.6. Gamo NJ, Arnsten AF. Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci. 2011; 125:282–296. PMID: 21480691.7. Wang DH, Wong-Lin K. Comodulation of dopamine and serotonin on prefrontal cortical rhythms: a theoretical study. Front Integr Nuerosci. 2013; 7:54.8. Di Pietro NC, Seamans JK. Dopamine and serotonin interactively modulate prefrontal cortex neurons in vitro . Biol Psychiatry. 2011; 69:1204–1211. PMID: 20889141.9. Salatino-Oliveira A, Rohde LA, Hutz MH. The dopamine transporter role in psychiatric phenotypes. Am J Med Genet B Neuropsychiatr Genet. 2018; 177:211–231. PMID: 28766921.10. Spies M, Knudsen GM, Lanzenberger R, Kasper S. The serotonin transporter in psychiatric disorders: insights from PET imaging. Lancet Psychiatry. 2015; 2:743–755. PMID: 26249305.11. Begum R, Howlader S, Mamun-Or-Rashid AN, Rafiquzzaman SM, Ashraf GM, Albadrani GM, Sayed AA, Peluso I, Abdel-Daim MM, Uddin MS. Antioxidant and signal-modulating effects of brown seaweed-derived compounds against oxidative stress-associated pathology. Oxid Med Cell Longev. 2021; 2021:9974890. PMID: 34336128.12. Um MY, Lim DW, Son HJ, Cho S, Lee C. Phlorotannin-rich fraction from Ishige foliacea brown seaweed prevents the scopolamine-induced memory impairment via regulation of ERK-CREB-BDNF pathway. J Funct Foods. 2018; 40:110–116.13. Guo F, Huang C, Cui Y, Momma H, Niu K, Nagatomi R. Dietary seaweed intake and depressive symptoms in Japanese adults: a prospective cohort study. Nutr J. 2019; 18:58. PMID: 31590668.14. Remya RR, Samrot AV, Kumar SS, Mohanavel V, Karthick A, Chinnaiyan VK, Umapathy D, Muhibbullah M. Bioactive potential of brown algae. Adsorpt Sci Technol. 2022; 2022:9104835.15. Kang SM, Cha SH, Ko JY, Kang MC, Kim D, Heo SJ, Kim JS, Heu MS, Kim YT, Jung WK, et al. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ Toxicol Pharmacol. 2012; 34:96–105. PMID: 22465981.16. Wu W, Han H, Liu J, Tang M, Wu X, Cao X, Zhao T, Lu Y, Niu T, Chen J, et al. Fucoxanthin prevents 6-OHDA-induced neurotoxicity by targeting Keap1. Oxid Med Cell Longev. 2021; 2021:6688708. PMID: 33777321.17. Meinita MD, Harwanto D, Sohn JH, Kim JS, Choi JS. Hizikia fusiformis: pharmacological and nutritional properties. Foods. 2021; 10:1660. PMID: 34359532.18. Seong SH, Nguyen DH, Wagle A, Woo MH, Jung HA, Choi JS. Experimental and computational study to reveal the potential of non-polar constituents from Hizikia fusiformis as dual protein tyrosine phosphatase 1B and α-glucosidase Inhibitors. Mar Drugs. 2019; 17:302. PMID: 31121891.19. Colella R, Lu C, Hodges B, Wilkey DW, Roisen FJ. GM1 enhances the association of neuron-specific MAP2 with actin in MAP2-transfected 3T3 cells. Brain Res Dev Brain Res. 2000; 121:1–9. PMID: 10837887.20. Schilling K, Barco EB, Rhinehart D, Pilgrim C. Expression of synaptophysin and neuron-specific enolase during neuronal differentiation in vitro: effects of dimethyl sulfoxide. J Neurosci Res. 1989; 24:347–354. PMID: 2512391.21. Yuan A, Rao MV, Veeranna , Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol. 2017; 9:a018309. PMID: 28373358.22. Niederkofler V, Asher TE, Dymecki SM. Functional interplay between dopaminergic and serotonergic neuronal systems during development and adulthood. ACS Chem Neurosci. 2015; 6:1055–1070. PMID: 25747116.23. Kang SY, Kim E, Kang I, Lee M, Lee Y. Anti-diabetic effects and anti-inflammatory effects of Laminaria japonica and Hizikia fusiforme in skeletal muscle: in vitro and in vivo model. Nutrients. 2018; 10:491. PMID: 29659527.24. Ling WH, Jones PJ. Dietary phytosterols: a review of metabolism, benefits and side effects. Life Sci. 1995; 57:195–206. PMID: 7596226.25. Hannan MA, Dash R, Sohag AAM, Moon IS. Deciphering molecular mechanism of the neuropharmacological action of fucosterol through integrated system pharmacology and in silico analysis. Mar Drugs. 2019; 17:639.26. Dimitrova-Shumkovska J, Krstanoski L, Veenman L. Potential beneficial actions of fucoidan in brain and liver injury, disease, and intoxication-potential implication of sirtuins. Mar Drugs. 2020; 18:242. PMID: 32380741.27. Peng J, Yuan JP, Wu CF, Wang JH. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Mar Drugs. 2011; 9:1806–1828. PMID: 22072997.28. Zhen XH, Quan YC, Jiang HY, Wen ZS, Qu YL, Guan LP. Fucosterol, a sterol extracted from Sargassum fusiforme, shows antidepressant and anticonvulsant effects. Eur J Pharmacol. 2015; 768:131–138. PMID: 26515446.29. Luo D, Zhang Q, Wang H, Cui Y, Sun Z, Yang J, Zheng Y, Jia J, Yu F, Wang X, et al. Fucoidan protects against dopaminergic neuron death in vivo and in vitro . Eur J Pharmacol. 2009; 617:33–40. PMID: 19545563.30. Paudel P, Seong SH, Jung HA, Choi JS. Characterizing fucoxanthin as a selective dopamine D3/D4 receptor agonist: relevance to Parkinson’s disease. Chem Biol Interact. 2019; 310:108757. PMID: 31323226.31. Song MY, Ku SK, Kim HJ, Han JS. Low molecular weight fucoidan ameliorating the chronic cisplatin-induced delayed gastrointestinal motility in rats. Food Chem Toxicol. 2012; 50:4468–4478. PMID: 23022014.32. Seong SH, Paudel P, Choi JW, Ahn DH, Nam TJ, Jung HA, Choi JS. Probing multi-target action of phlorotannins as new monoamine oxidase inhibitors and dopaminergic receptor modulators with the potential for treatment of neuronal disorders. Mar Drugs. 2019; 17:377. PMID: 31238535.33. Artiges E, Leroy C, Dubol M, Prat M, Pepin A, Mabondo A, de Beaurepaire R, Beaufils B, Korwin JP, Galinowski A, et al. Striatal and extrastriatal dopamine transporter availability in schizophrenia and its clinical correlates: a voxel-based and high-resolution PET study. Schizophr Bull. 2017; 43:1134–1142. PMID: 28177089.34. Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, Tsuchiya KJ, Sugihara G, Iwata Y, Suzuki K, et al. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry. 2010; 67:59–68. PMID: 20048223.35. Pizzagalli DA, Berretta S, Wooten D, Goer F, Pilobello KT, Kumar P, Murray L, Beltzer M, Boyer-Boiteau A, Alpert N, et al. Assessment of striatal dopamine transporter binding in individuals with major depressive disorder: in vivo positron emission tomography and postmortem evidence. JAMA Psychiatry. 2019; 76:854–861. PMID: 31042280.36. Tiger M, Varnäs K, Okubo Y, Lundberg J. The 5-HT1B receptor - a potential target for antidepressant treatment. Psychopharmacology (Berl). 2018; 235:1317–1334. PMID: 29546551.37. Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019; 90:870–881. PMID: 30967444.38. Zhang L, Bai J, Undie AS, Bergson C, Lidow MS. D1 dopamine receptor regulation of the levels of the cell-cycle-controlling proteins, cyclin D, P27 and Raf-1, in cerebral cortical precursor cells is mediated through cAMP-independent pathways. Cereb Cortex. 2005; 15:74–84. PMID: 15238444.39. Song ZM, Undie AS, Koh PO, Fang YY, Zhang L, Dracheva S, Sealfon SC, Lidow MS. D1 dopamine receptor regulation of microtubule-associated protein-2 phosphorylation in developing cerebral cortical neurons. J Neurosci. 2002; 22:6092–6105. PMID: 12122070.40. Lotto B, Upton L, Price DJ, Gaspar P. Serotonin receptor activation enhances neurite outgrowth of thalamic neurones in rodents. Neurosci Lett. 1999; 269:87–90. PMID: 10430511.41. Ji B, Higa K, Soontornniyomkij V, Miyanohara A, Zhou X. A novel animal model for neuroinflammation and white matter degeneration. PeerJ. 2017; 5:e3905. PMID: 29104820.42. Ehlers MD, Fung ET, O’Brien RJ, Huganir RL. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci. 1998; 18:720–730. PMID: 9425014.43. Kim OJ, Ariano MA, Lazzarini RA, Levine MS, Sibley DR. Neurofilament-M interacts with the D1 dopamine receptor to regulate cell surface expression and desensitization. J Neurosci. 2002; 22:5920–5930. PMID: 12122054.44. Diekämper E, Brix B, Stöcker W, Vielhaber S, Galazky I, Kreissl MC, Genseke P, Düzel E, Körtvelyessy P. Neurofilament levels are reflecting the loss of presynaptic dopamine receptors in movement disorders. Front Neurosci. 2021; 15:690013. PMID: 34924923.45. Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000; 417:181–194. PMID: 10660896.46. Hjorth S, Suchowski CS, Galloway MP. Evidence for 5-HT autoreceptor-mediated, nerve impulse-independent, control of 5-HT synthesis in the rat brain. Synapse. 1995; 19:170–176. PMID: 7784957.47. Hagan CE, McDevitt RA, Liu Y, Furay AR, Neumaier JF. 5-HT(1B) autoreceptor regulation of serotonin transporter activity in synaptosomes. Synapse. 2012; 66:1024–1034. PMID: 22961814.48. Hou H, Tian M, Zhang H. Positron emission tomography molecular imaging of dopaminergic system in drug addiction. Anat Rec (Hoboken). 2012; 295:722–733. PMID: 22467195.49. Gryglewski G, Lanzenberger R, Kranz GS, Cumming P. Meta-analysis of molecular imaging of serotonin transporters in major depression. J Cereb Blood Flow Metab. 2014; 34:1096–1103. PMID: 24802331.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Polysaccharide from Hizikia Fusiformis Enhances the Immunomodulatory Activity of Macrophages
- Extracellular HMGB1 Released after Zinc-treatment Induces Neuronal Death in Primary Cortical Cultures
- Study on the Protective Effects of 6R-Tetrahydrobiopterin on the Oxidative Neuronal Injury in Mouse Cortical Cultures
- Role of Serotonin in Pathophysiology and Treatment of Schizophrenia
- Both R(-)- and S(+)-Citalopram Attenuate Neuronal Apoptosis in Murine Cortical Cell Culture