J Korean Med Sci.
2023 Apr;38(14):e109. 10.3346/jkms.2023.38.e109.
Anti-S1/RBD-Specific Antibody Formation After SARS-CoV-2 Vaccination in Elderly Rheumatoid Arthritis Patients: Single-Center Prospective Observational Study
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Division of Rheumatology, Department of Internal Medicine, Korea University Ansan Hospital, Seoul, Korea
- 3Department of Information Medicine, Big Data Research Center, Asan Medical Center, Seoul, Korea
- 4Convergence Medicine Research Center, Asan Institute for Life Science, Asan Medical Center, Seoul, Korea
- KMID: 2541550
- DOI: http://doi.org/10.3346/jkms.2023.38.e109
Abstract
- Background
The guidelines of coronavirus disease 2019 (COVID-19) vaccination in patients with rheumatoid arthritis (RA) have been continuously updated, with extensive discussion on the effectiveness of the COVID-19 booster vaccines and antibody generation associated with the different types of vaccine. We investigated the effects of the third dose of the mRNA vaccine on antibody titer and the factors associated with antibody production in patients with RA who had previously received two doses of the ChAdOx1-S nCoV-19 vaccine.
Methods
Between October 14, 2021 and June 17, 2022, two patient groups diagnosed with RA were recruited prospectively: one with two doses of ChAdOx1-S nCoV-19 and the second group with the additional third mRNA vaccine. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody titers were determined through semiquantitative anti-SARS-CoV-2 spike (S) electrochemiluminescence immunoassay. Antibody titers were compared in both groups considering clinical features and medications. Multivariate logistic regression was performed to identify the factors associated with antibody production. Also, we followed up the antibody titers of whom completed the 3rd mRNA vaccination.
Results
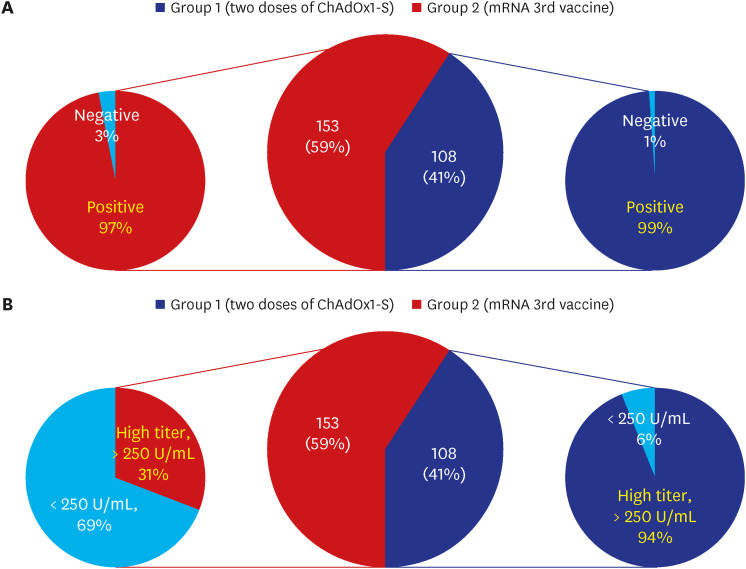
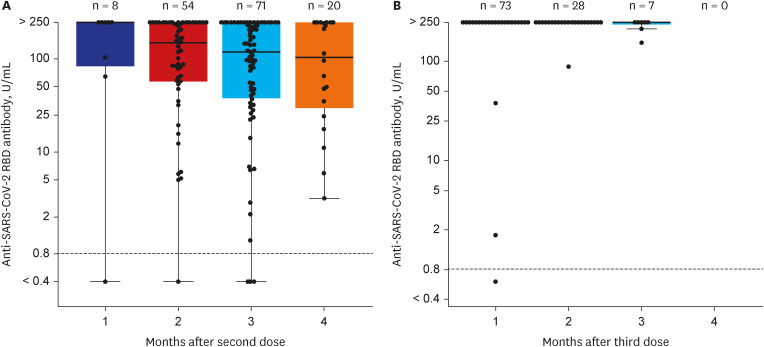
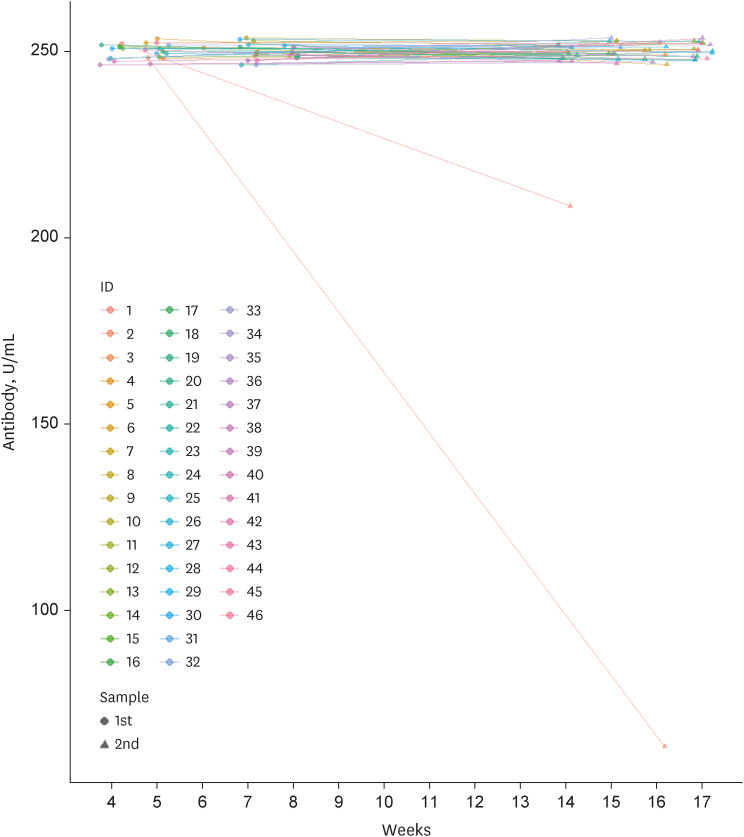
Among 261 patients, all patients were over 60 years old except for 7 patients and the average age was 65 years; 153 had completed two doses of ChAdOx1-S nCoV-19, while 108 patients had also received the third mRNA vaccine. The positive rates of anti-SARS-CoV-2 anti-S1/receptor binding domain-specific antibody (titer > 0.8 U/mL) were 97% (149/153) and 99% (107/108) respectively. However, positive rates for high antibody titer (> 250 U/ mL) were found in only 31% (47/153) of group 1 but 94% (102/108) of group 2. Multivariate analysis revealed that corticosteroid use (odds ratio [OR], 0.35; 95% confidence interval [CI], 0.16–0.75), older age (OR, 0.91; 95% CI, 0.860–0.98), and male sex (OR, 0.23; 95% CI, 0.07–0.74) were associated with a lower rate of high antibody titer acquisition after two doses of ChAdOx1-S nCoV-19. Waning of antibody titers was observed in only two of 46 patients who followed up twice after the third mRNA vaccine inoculation.
Conclusion
Our findings suggest that the third dose of the mRNA vaccine could be beneficial in RA patients with risk factors including older age, male sex, and corticosteroid use after two doses of ChAdOx1-S nCoV-19.
Figure
Reference
-
1. Day AL, Winthrop KL, Curtis JR. The effect of disease-modifying antirheumatic drugs on vaccine immunogenicity in adults. Cleve Clin J Med. 2020; 87(11):695–703. PMID: 33139263.2. Jani M, Barton A, Warren RB, Griffiths CE, Chinoy H. The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology (Oxford). 2014; 53(2):213–222. PMID: 23946436.3. Schaeverbeke T, Truchetet ME, Kostine M, Barnetche T, Bannwarth B, Richez C. Immunogenicity of biologic agents in rheumatoid arthritis patients: lessons for clinical practice. Rheumatology (Oxford). 2016; 55(2):210–220. PMID: 26268816.4. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384(5):403–416. PMID: 33378609.5. Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021; 397(10269):99–111. PMID: 33306989.6. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.7. Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020; 383(25):2439–2450. PMID: 33053279.8. Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021; 384(1):80–82. PMID: 33270381.9. Boyarsky BJ, Ruddy JA, Connolly CM, Ou MT, Werbel WA, Garonzik-Wang JM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021; 80(8):1098–1099. PMID: 33757968.10. Connolly CM, Boyarsky BJ, Ruddy JA, Werbel WA, Christopher-Stine L, Garonzik-Wang JM, et al. Absence of humoral response after two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases: a case series. Ann Intern Med. 2021; 174(9):1332–1334. PMID: 34029488.
Article11. Winthrop KL, Bingham CO 3rd, Komocsar WJ, Bradley J, Issa M, Klar R, et al. Evaluation of pneumococcal and tetanus vaccine responses in patients with rheumatoid arthritis receiving baricitinib: results from a long-term extension trial substudy. Arthritis Res Ther. 2019; 21(1):102. PMID: 30999933.12. Ammitzbøll C, Bartels LE, Bøgh Andersen J, Risbøl Vils S, Elbaek Mistegård C, Dahl Johannsen A, et al. Impaired antibody response to the BNT162b2 messenger RNA coronavirus disease 2019 vaccine in patients with systemic lupus erythematosus and rheumatoid arthritis. ACR Open Rheumatol. 2021; 3(9):622–628. PMID: 34273260.13. Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for COVID-19. N Engl J Med. 2021; 384(23):2259–2261. PMID: 33822494.14. Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021; 397(10269):72–74. PMID: 33306990.15. Gobbi F, Buonfrate D, Moro L, Rodari P, Piubelli C, Caldrer S, et al. Antibody response to the BNT162b2 mRNA COVID-19 vaccine in subjects with prior SARS-CoV-2 infection. Viruses. 2021; 13(3):422. PMID: 33807957.
Article16. Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (Oxford). 2012; 51(Suppl 6):vi5–vi9. PMID: 23221588.17. Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009; 68(6):954–960. PMID: 18490431.18. Park JK, Lee EB, Shin K, Sung YK, Kim TH, Kwon SR, et al. COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: clinical guidance of the Korean College of Rheumatology. J Korean Med Sci. 2021; 36(12):e95. PMID: 33783147.
Article19. Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021; 384(6):533–540. PMID: 33369366.20. Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021; 27(2):205–211. PMID: 33469205.21. Wheatley AK, Juno JA, Wang JJ, Selva KJ, Reynaldi A, Tan HX, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun. 2021; 12(1):1162. PMID: 33608522.22. Gaebler C, Wang Z, Lorenzi JC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021; 591(7851):639–644. PMID: 33461210.23. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021; 371(6529):eabf4063. PMID: 33408181.24. Ruddy JA, Connolly CM, Boyarsky BJ, Werbel WA, Christopher-Stine L, Garonzik-Wang J, et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021; 80(10):1351–1352. PMID: 34031032.25. Chiang TP, Connolly CM, Ruddy JA, Boyarsky BJ, Alejo JL, Werbel WA, et al. Antibody response to the Janssen/Johnson & Johnson SARS-CoV-2 vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021; 80(10):1365–1366. PMID: 34429320.26. Kang YM, Minn D, Lim J, Lee KD, Jo DH, Choe KW, et al. Comparison of antibody response elicited by ChAdOx1 and BNT162b2 COVID-19 vaccine. J Korean Med Sci. 2021; 36(46):e311. PMID: 34845875.27. Ji W, Huh K, Kang M, Hong J, Bae GH, Lee R, et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020; 35(25):e237. PMID: 32597048.28. Kim DW, Byeon KH, Kim J, Cho KD, Lee N. The correlation of comorbidities on the mortality in patients with COVID-19: an observational study based on the Korean National Health Insurance Big Data. J Korean Med Sci. 2020; 35(26):e243. PMID: 32627443.29. Soegiarto G, Wulandari L, Purnomosari D, Dhia Fahmita K, Ikhwan Gautama H, Tri Hadmoko S, et al. Hypertension is associated with antibody response and breakthrough infection in health care workers following vaccination with inactivated SARS-CoV-2. Vaccine. 2022; 40(30):4046–4056. PMID: 35660034.30. Alexander JL, Kennedy NA, Ibraheim H, Anandabaskaran S, Saifuddin A, Castro Seoane R, et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepatol. 2022; 7(4):342–352. PMID: 35123676.31. Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020; 39(7):2085–2094. PMID: 32474885.32. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020; 117(20):10970–10975. PMID: 32350134.33. Mori S, Ueki Y, Hirakata N, Oribe M, Hidaka T, Oishi K. Impact of tocilizumab therapy on antibody response to influenza vaccine in patients with rheumatoid arthritis. Ann Rheum Dis. 2012; 71(12):2006–2010. PMID: 22887851.34. Bingham CO 3rd, Rizzo W, Kivitz A, Hassanali A, Upmanyu R, Klearman M. Humoral immune response to vaccines in patients with rheumatoid arthritis treated with tocilizumab: results of a randomised controlled trial (VISARA). Ann Rheum Dis. 2015; 74(5):818–822. PMID: 24448345.35. Tsuru T, Terao K, Murakami M, Matsutani T, Suzaki M, Amamoto T, et al. Immune response to influenza vaccine and pneumococcal polysaccharide vaccine under IL-6 signal inhibition therapy with tocilizumab. Mod Rheumatol. 2014; 24(3):511–516. PMID: 24252023.36. Kellam P, Barclay W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J Gen Virol. 2020; 101(8):791–797. PMID: 32430094.37. Choe PG, Kang CK, Suh HJ, Jung J, Song KH, Bang JH, et al. Waning antibody responses in asymptomatic and symptomatic SARS-CoV-2 infection. Emerg Infect Dis. 2021; 27(1):327–329. PMID: 33050983.38. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021; 385(24):e84. PMID: 34614326.39. Liu P, Cai J, Jia R, Xia S, Wang X, Cao L, et al. Dynamic surveillance of SARS-CoV-2 shedding and neutralizing antibody in children with COVID-19. Emerg Microbes Infect. 2020; 9(1):1254–1258. PMID: 32515685.40. Spellberg B, Nielsen TB, Casadevall A. Antibodies, immunity, and COVID-19. JAMA Intern Med. 2021; 181(4):460–462. PMID: 33231673.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity of SARS-CoV-2 vaccine in kidney transplant recipients: a cross-sectional study in Korea
- Clinical Usefulness of SARS-CoV-2 Antibody Test
- Immunogenicity of SARS-CoV-2 Vaccine in Kidney Transplant Recipients: A Cross-Sectional Study in Korea
- Comparison of the rapidity of SARS-CoV-2 immune responses between primary and booster vaccination for COVID-19
- Recombinant proteins of spike protein of SARS-CoV-2 with the Omicron receptor-binding domain induce production of highly Omicron-specific neutralizing antibodies