Korean J Physiol Pharmacol.
2023 Jan;27(1):9-20. 10.4196/kjpp.2023.27.1.9.
Hyperbaric oxygenation applied before or after mild or hard stress: effects on the redox state in the muscle tissue
- Affiliations
-
- 1Escuela Superior de Medicina, Sección de Estudio de Posgrado e Investigación, Instituto Politécnico Nacional, Mexico City 11340, Mexico
- KMID: 2537499
- DOI: http://doi.org/10.4196/kjpp.2023.27.1.9
Abstract
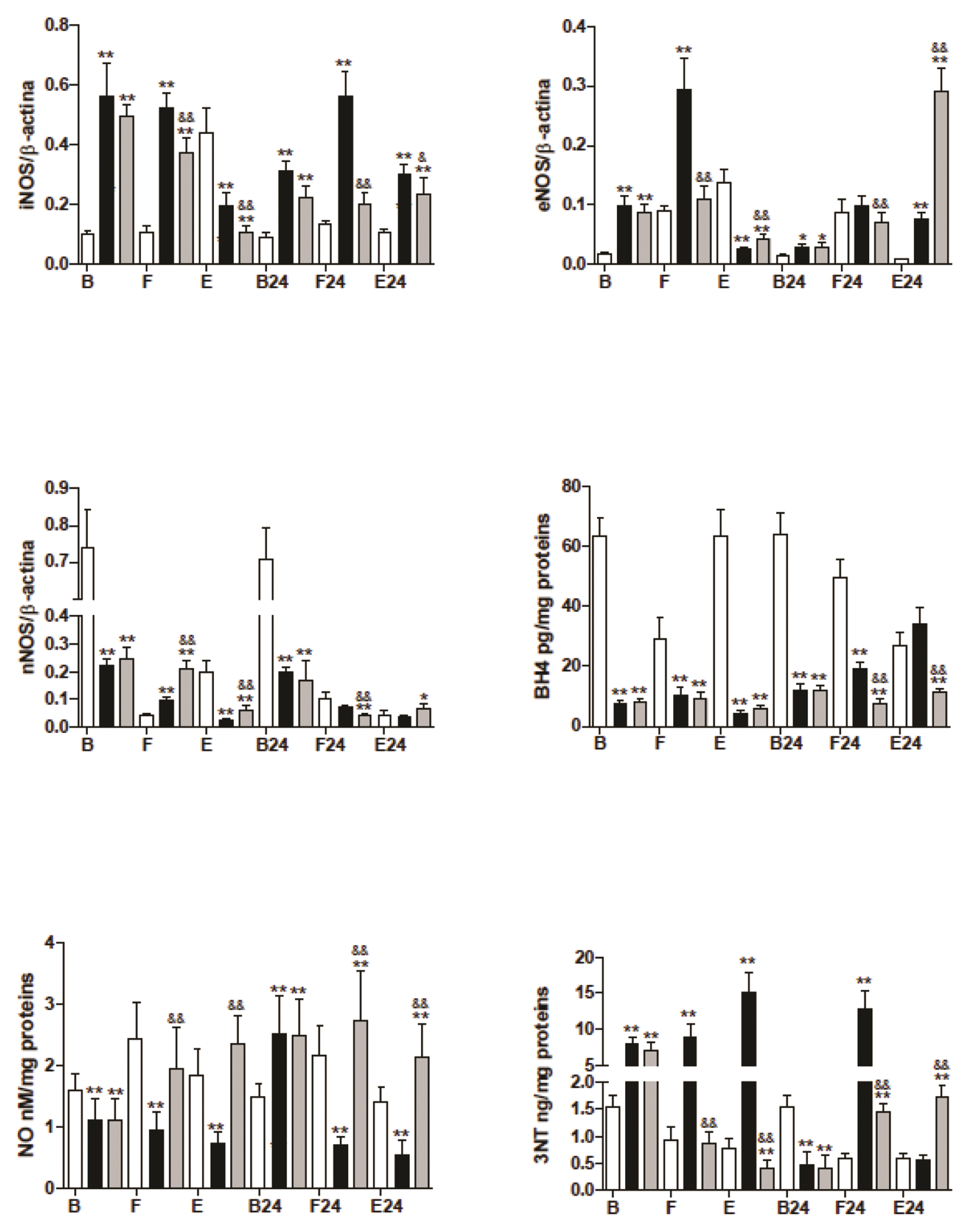
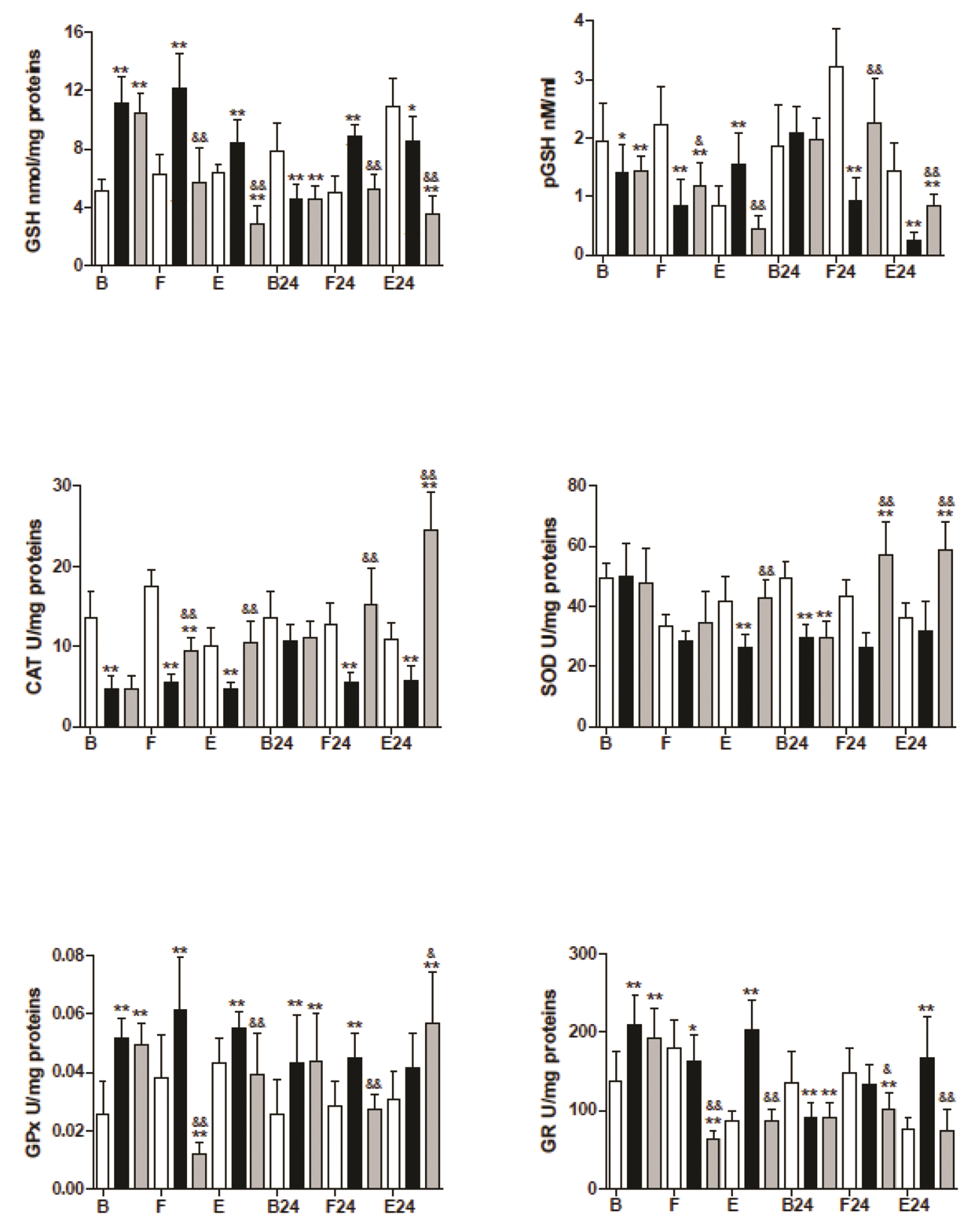
- The mechanism is unclear for the reported protective effect of hyperbaric oxygen preconditioning against oxidative stress in tissues, and the distinct effects of hyperbaric oxygen applied after stress. The trained mice were divided into three groups: the control, hyperbaric oxygenation preconditioning, and hyperbaric oxygenation applied after mild (fasting) or hard (prolonged exercise) stress. After preconditioning, we observed a decrease in basal levels of nitric oxide, tetrahydrobiopterin, and catalase despite the drastic increase in inducible and endothelial nitric oxide synthases. Moreover, the basal levels of glutathione, related enzymes, and nitrosative stress only increased in the preconditioning group. The control and preconditioning groups showed a similar mild stress response of the endothelial and neuronal nitric oxide synthases. At the same time, the activity of all nitric oxide synthase, glutathione (GSH) in muscle, declined in the experimental groups but increased in control during hard stress. The results suggested that hyperbaric oxygen preconditioning provoked uncoupling of nitric oxide synthases and the elevated levels of GSH in muscle during this study, while hyperbaric oxygen applied after stress showed a lower level of GSH but higher recovery post-exercise levels in the majority of antioxidant enzymes. We discuss the possible mechanisms of the redox response and the role of the nitric oxide in this process.
Keyword
Figure
Reference
-
1. Yamamoto N, Oyaizu T, Enomoto M, Horie M, Yuasa M, Okawa A, Yagishita K. 2020; VEGF and bFGF induction by nitric oxide is associated with hyperbaric oxygen-induced angiogenesis and muscle regeneration. Sci Rep. 10:2744. DOI: 10.1038/s41598-020-59615-x. PMID: 32066777. PMCID: PMC7026099. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85079660139&origin=inward.2. Fernando V, Zheng X, Walia Y, Sharma V, Letson J, Furuta S. 2019; S-nitrosylation: an emerging paradigm of redox signaling. Antioxidants (Basel). 8:404. DOI: 10.3390/antiox8090404. PMID: 31533268. PMCID: PMC6769533. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85073441694&origin=inward.3. Yogaratnam JZ, Laden G, Guvendik L, Cowen M, Cale A, Griffin S. 2008; Pharmacological preconditioning with hyperbaric oxygen: can this therapy attenuate myocardial ischemic reperfusion injury and induce myocardial protection via nitric oxide? J Surg Res. 149:155–164. DOI: 10.1016/j.jss.2007.09.003. PMID: 17996900. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=48949101711&origin=inward.4. Liu W, Li J, Sun X, Liu K, Zhang JH, Xu W, Tao H. 2008; Repetitive hyperbaric oxygen exposures enhance sensitivity to convulsion by upregulation of eNOS and nNOS. Brain Res. 1201:128–134. DOI: 10.1016/j.brainres.2008.01.068. PMID: 18342297. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=40849107758&origin=inward.5. Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. 2003; Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 278:22546–22554. DOI: 10.1074/jbc.M302227200. PMID: 12692136. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0038604446&origin=inward.6. Luo S, Lei H, Qin H, Xia Y. 2014; Molecular mechanisms of endothelial NO synthase uncoupling. Curr Pharm Des. 20:3548–3553. DOI: 10.2174/13816128113196660746. PMID: 24180388. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84906794400&origin=inward.7. Baldelli S, Ciccarone F, Limongi D, Checconi P, Palamara AT, Ciriolo MR. 2019; Glutathione and nitric oxide: key team players in use and disuse of skeletal muscle. Nutrients. 11:2318. DOI: 10.3390/nu11102318. PMID: 31575008. PMCID: PMC6836164. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85072847717&origin=inward.8. Payabvash S, Ghahremani MH, Goliaei A, Mandegary A, Shafaroodi H, Amanlou M, Dehpour AR. 2006; Nitric oxide modulates glutathione synthesis during endotoxemia. Free Radic Biol Med. 41:1817–1828. DOI: 10.1016/j.freeradbiomed.2006.09.010. PMID: 17157184. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33751545345&origin=inward.9. Feng Y, Zhang Z, Li Q, Li W, Xu J, Cao H. 2015; Hyperbaric oxygen preconditioning protects lung against hyperoxic acute lung injury in rats via heme oxygenase-1 induction. Biochem Biophys Res Commun. 456:549–554. DOI: 10.1016/j.bbrc.2014.09.074. PMID: 25264201. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84919883782&origin=inward.10. Gao ZX, Rao J, Li YH. 2017; Hyperbaric oxygen preconditioning improves postoperative cognitive dysfunction by reducing oxidant stress and inflammation. Neural Regen Res. 12:329–336. DOI: 10.4103/1673-5374.200816. PMID: 28400818. PMCID: PMC5361520. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85014921063&origin=inward.11. Kormanovski A, del Carmen Castillo-Hernández M, Guevara-Balcázar G, Pérez T, Lara-Padilla E. 2019; Gender differences in nitric oxide and antioxidant response to physical stress in tissues of trained mice. Physiol Pharmacol. 23:224–234.12. Kormanovski A, del Carmen Castillo-Hernández M, Guevara-Balcázar G, Pérez T, Lara-Padilla E. 2019; Gender differences in nitric oxide and antioxidant response to physical stress in tissues of trained mice after hyperbaric oxygen preconditioning. Physiol Pharmacol. 23:309–321.13. Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, et al. 2020; The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18:e3000410. DOI: 10.1371/journal.pbio.3000410. PMID: 32663219. PMCID: PMC7360023. PMID: 143566b401ed41fd9517d9c18b875cd9. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85087822452&origin=inward.14. Crimi E, Ignarro LJ, Cacciatore F, Napoli C. 2009; Mechanisms by which exercise training benefits patients with heart failure. Nat Rev Cardiol. 6:292–300. DOI: 10.1038/nrcardio.2009.8. PMID: 19352333. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=67649644862&origin=inward.15. Roof SR, Tang L, Ostler JE, Periasamy M, Györke S, Billman GE, Ziolo MT. 2013; Neuronal nitric oxide synthase is indispensable for the cardiac adaptive effects of exercise. Basic Res Cardiol. 108:332. DOI: 10.1007/s00395-013-0332-6. PMID: 23377961. PMCID: PMC3575690. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84873373133&origin=inward.16. Roof SR, Ho HT, Little SC, Ostler JE, Brundage EA, Periasamy M, Villamena FA, Györke S, Biesiadecki BJ, Heymes C, Houser SR, Davis JP, Ziolo MT. 2015; Obligatory role of neuronal nitric oxide synthase in the heart's antioxidant adaptation with exercise. J Mol Cell Cardiol. 81:54–61. DOI: 10.1016/j.yjmcc.2015.01.003. PMID: 25595735. PMCID: PMC4380650. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84922361434&origin=inward.17. Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. 2003; Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 111:1201–1209. DOI: 10.1172/JCI200314172. PMID: 12697739. PMCID: PMC152929. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037399439&origin=inward.18. Rubio-Guerra AF, Vargas-Robles H, Ramos-Brizuela LM, Escalante-Acosta BA. 2010; Is tetrahydrobiopterin a therapeutic option in diabetic hypertensive patients? Integr Blood Press Control. 3:125–132. DOI: 10.2147/IBPC.S7479. PMID: 21949628. PMCID: PMC3172060.19. Ganzarolli de Oliveira M. 2016; S-nitrosothiols as platforms for topical nitric oxide delivery. Basic Clin Pharmacol Toxicol. 119 Suppl 3:49–56. DOI: 10.1111/bcpt.12588. PMID: 27030007. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84992647008&origin=inward.20. Crabtree MJ, Brixey R, Batchelor H, Hale AB, Channon KM. 2013; Integrated redox sensor and effector functions for tetrahydrobiopterin- and glutathionylation-dependent endothelial nitric-oxide synthase uncoupling. J Biol Chem. 288:561–569. DOI: 10.1074/jbc.M112.415992. PMID: 23139420. PMCID: PMC3537053. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84872094899&origin=inward.21. Singh SP, Wishnok JS, Keshive M, Deen WM, Tannenbaum SR. 1996; The chemistry of the S-nitrosoglutathione/glutathione system. Proc Natl Acad Sci U S A. 93:14428–14433. DOI: 10.1073/pnas.93.25.14428. PMID: 8962068. PMCID: PMC26149. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029856045&origin=inward.22. Nasr R, Lorendeau D, Khonkarn R, Dury L, Pérès B, Boumendjel A, Cortay JC, Falson P, Chaptal V, Baubichon-Cortay H. 2020; Molecular analysis of the massive GSH transport mechanism mediated by the human Multidrug Resistant Protein 1/ABCC1. Sci Rep. 10:7616. DOI: 10.1038/s41598-020-64400-x. PMID: 32377003. PMCID: PMC7203140. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85084404519&origin=inward.23. Ballatori N, Krance SM, Marchan R, Hammond CL. 2009; Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol Aspects Med. 30:13–28. DOI: 10.1016/j.mam.2008.08.004. PMID: 18786560. PMCID: PMC2716123. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=65049090568&origin=inward.24. Kuo PC, Abe KY, Schroeder RA. 1996; Interleukin-1-induced nitric oxide production modulates glutathione synthesis in cultured rat hepatocytes. Am J Physiol. 271(3 Pt 1):C851–C862. DOI: 10.1152/ajpcell.1996.271.3.C851. PMID: 8843715. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029819251&origin=inward.25. Moellering D, Mc Andrew J, Patel RP, Forman HJ, Mulcahy RT, Jo H, Darley-Usmar VM. 1999; The induction of GSH synthesis by nanomolar concentrations of NO in endothelial cells: a role for gamma-glutamylcysteine synthetase and gamma-glutamyl transpeptidase. FEBS Lett. 448:292–296. DOI: 10.1016/S0014-5793(99)00371-3. PMID: 10218495. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0032951247&origin=inward.26. Minamiyama Y, Takemura S, Koyama K, Yu H, Miyamoto M, Inoue M. 1996; Dynamic aspects of glutathione and nitric oxide metabolism in endotoxemic rats. Am J Physiol. 271(4 Pt 1):G575–G581. DOI: 10.1152/ajpgi.1996.271.4.G575. PMID: 8897875. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029862130&origin=inward.27. Moellering D, McAndrew J, Patel RP, Cornwell T, Lincoln T, Cao X, Messina JL, Forman HJ, Jo H, Darley-Usmar VM. 1998; Nitric oxide-dependent induction of glutathione synthesis through increased expression of gamma-glutamylcysteine synthetase. Arch Biochem Biophys. 358:74–82. DOI: 10.1006/abbi.1998.0854. PMID: 9750167. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0032190207&origin=inward.28. Liu RM, Hu H, Robison TW, Forman HJ. 1996; Increased gamma-glutamylcysteine synthetase and gamma-glutamyl transpeptidase activities enhance resistance of rat lung epithelial L2 cells to quinone toxicity. Am J Respir Cell Mol Biol. 14:192–197. DOI: 10.1165/ajrcmb.14.2.8630270. PMID: 8630270. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0030077954&origin=inward.29. Kahraman S, Düz B, Kayali H, Korkmaz A, Oter S, Aydin A, Sayal A. 2007; Effects of methylprednisolone and hyperbaric oxygen on oxidative status after experimental spinal cord injury: a comparative study in rats. Neurochem Res. 32:1547–1551. DOI: 10.1007/s11064-007-9354-5. PMID: 17486444. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34447629751&origin=inward.30. Cheng O, Ostrowski RP, Wu B, Liu W, Chen C, Zhang JH. 2011; Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke. 42:484–490. DOI: 10.1161/STROKEAHA.110.604421. PMID: 21164135. PMCID: PMC3026922. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79251643147&origin=inward.31. Fuller AM, Giardina C, Hightower LE, Perdrizet GA, Tierney CA. 2013; Hyperbaric oxygen preconditioning protects skin from UV-A damage. Cell Stress Chaperones. 18:97–107. DOI: 10.1007/s12192-012-0362-2. PMID: 22855227. PMCID: PMC3508122. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84870474066&origin=inward.32. Nathan C, Xie QW. 1994; Nitric oxide synthases: roles, tolls, and controls. Cell. 78:915–918. DOI: 10.1016/0092-8674(94)90266-6. PMID: 7522969. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0028092958&origin=inward.33. Bendall JK, Douglas G, McNeill E, Channon KM, Crabtree MJ. 2014; Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal. 20:3040–3077. DOI: 10.1089/ars.2013.5566. PMID: 24294830. PMCID: PMC4038990. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84901638616&origin=inward.34. Feng Y, Feng Y, Gu L, Liu P, Cao J, Zhang S. 2021; The critical role of tetrahydrobiopterin (BH4) metabolism in modulating radiosensitivity: BH4/NOS axis as an angel or a devil. Front Oncol. 11:1–12. DOI: 10.3389/fonc.2021.720632. PMID: 34513700. PMCID: PMC8429800. PMID: d17a6770c99c4a2db3fd154e45cdbd0b. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85114748250&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An experimental study on the change of EKG in hyperbaric oxygenation
- Free Flap Coverage for Radiation Ulcer on Lumbosacral Region
- Treatment of radiation-induced cystitis with hyperbaric oxygen
- A Clinical Study of Hyperbaric Oxygenation Therapy
- A study on the Effects of Hyperbaric Oxygenation Combined with the Drug Administration in the Treatment of CO poisoning