Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach
- Affiliations
-
- 1Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Pathology, Kameda Medical Center, Kamogawa, Japan
- 4Department of Pathology, Cancer Genome Center and Thyroid Disease Center, Izumi City General Hospital, Izumi, Japan
- KMID: 2534623
- DOI: http://doi.org/10.3803/EnM.2022.1553
Abstract
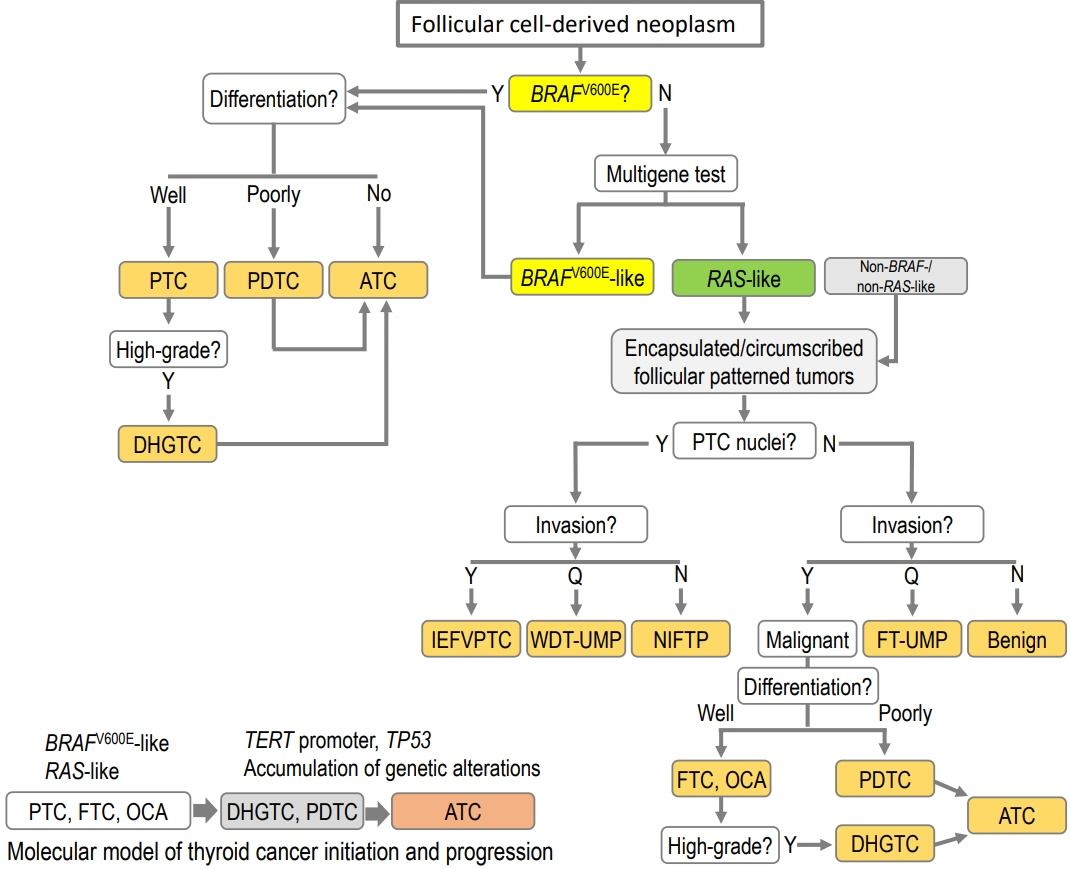
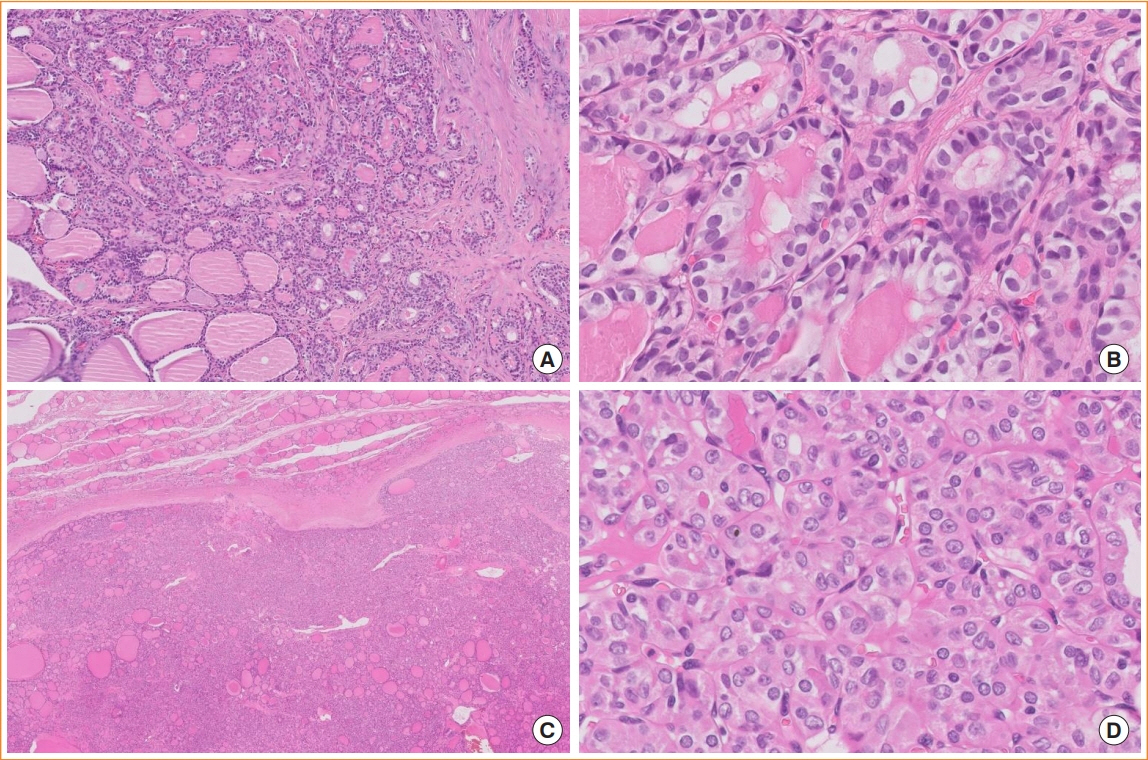
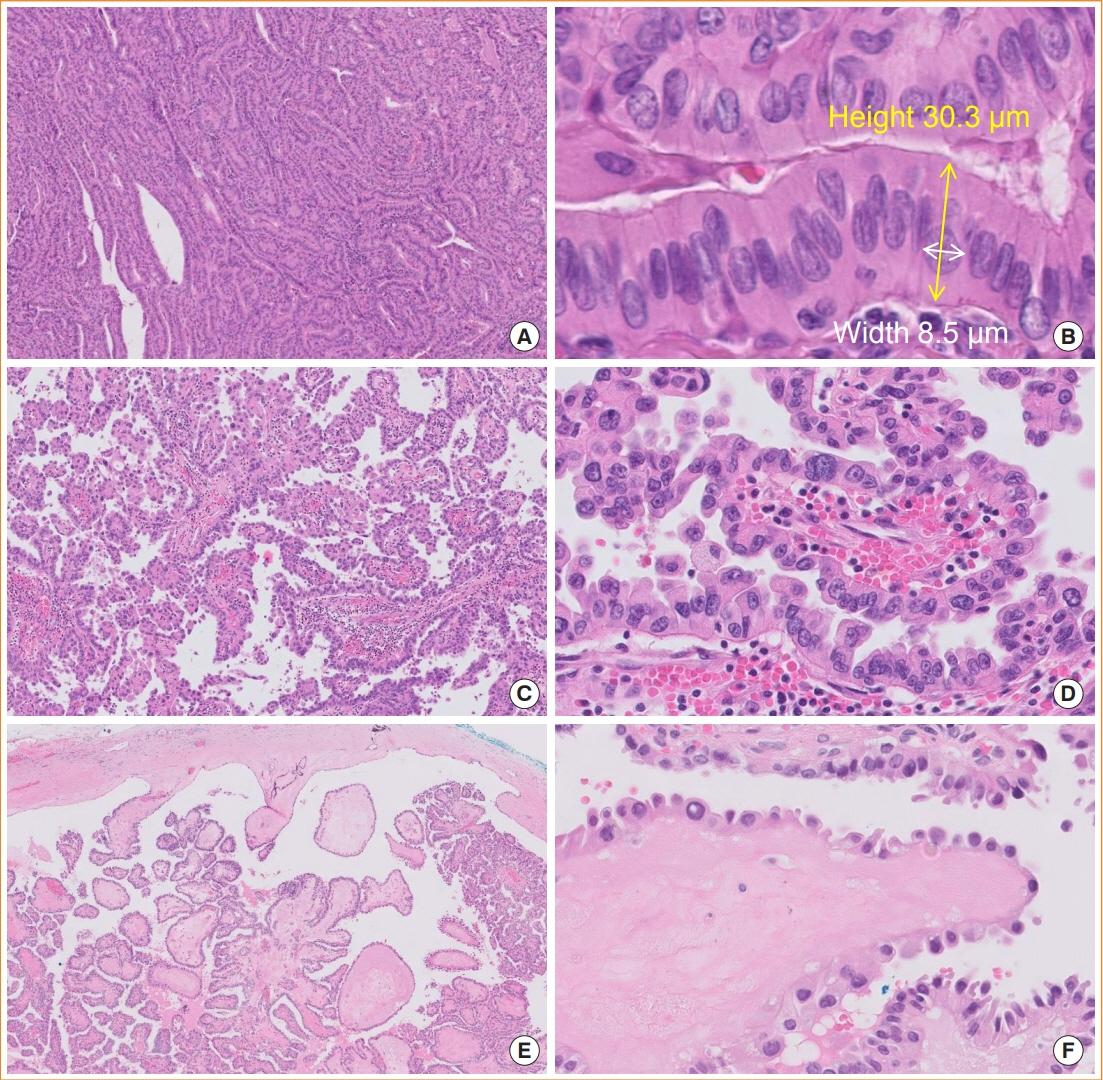
- The fifth edition of the World Health Organization (WHO) histologic classification of thyroid neoplasms released in 2022 includes newly recognized tumor types, subtypes, and a grading system. Follicular cell-derived neoplasms are categorized into three families (classes): benign tumors, low-risk neoplasms, and malignant neoplasms. The terms “follicular nodular disease” and “differentiated high-grade thyroid carcinoma” are introduced to account for multifocal hyperplastic/neoplastic lesions and differentiated thyroid carcinomas with high-grade features, respectively. The term “Hürthle cells” is replaced with “oncocytic cells.” Invasive encapsulated follicular and cribriform morular variants of papillary thyroid carcinoma (PTC) are now redefined as distinct tumor types, given their different genetic alterations and clinicopathologic characteristics from other PTC subtypes. The term “variant” to describe a subclass of tumor has been replaced with the term “subtype.” Instead, the term “variant” is reserved to describe genetic alterations. A histologic grading system based on the mitotic count, necrosis, and/or the Ki67 index is used to identify high-grade follicular-cell derived carcinomas and medullary thyroid carcinomas. The 2022 WHO classification introduces the following new categories: “salivary gland-type carcinomas of the thyroid” and “thyroid tumors of uncertain histogenesis.” This review summarizes the major changes in the 2022 WHO classification and their clinical relevance.
Figure
Cited by 4 articles
-
The Asian Thyroid Working Group, from 2017 to 2023
Kennichi Kakudo, Chan Kwon Jung, Zhiyan Liu, Mitsuyoshi Hirokawa, Andrey Bychkov, Huy Gia Vuong, Somboon Keelawat, Radhika Srinivasan, Jen-Fan Hang, Chiung-Ru Lai
J Pathol Transl Med. 2023;57(6):289-304. doi: 10.4132/jptm.2023.10.04.Korean Thyroid Association Guidelines on the Management of Differentiated Thyroid Cancers; Part I. Initial Management of Differentiated Thyroid Cancers - Chapter 4. Pathological Diagnosis and Staging after Thyroidectomy 2024
Su-Jin Shin, Hee Young Na, Ho-Cheol Kang, Sun Wook Kim, Dong Gyu Na, Young Joo Park, Young Shin Song, Eun Kyung Lee, Dong-Jun Lim, Yun Jae Chung, Chan Kwon Jung
Int J Thyroidol. 2024;17(1):61-67. doi: 10.11106/ijt.2024.17.1.61.Utilizing Immunoglobulin G4 Immunohistochemistry for Risk Stratification in Patients with Papillary Thyroid Carcinoma Associated with Hashimoto Thyroiditis
Faridul Haq, Gyeongsin Park, Sora Jeon, Mitsuyoshi Hirokawa, Chan Kwon Jung
Endocrinol Metab. 2024;39(3):468-478. doi: 10.3803/EnM.2024.1923.Prognosis of Poorly Differentiated Thyroid Carcinoma: A Systematic Review and Meta-Analysis
Ji Young Kim, Jae Kyung Myung, Soyun Kim, Kyung Tae, Yun Young Choi, Soo Jin Lee
Endocrinol Metab. 2024;39(4):590-602. doi: 10.3803/EnM.2024.1927.
Reference
-
1. Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs. 4th ed. Lyon: International Agency for Research on Cancer (IARC);2017. p. 65–91.2. Mete O. Special issue on the 2022 WHO classification of endocrine and neuroendocrine tumors: a new primer for endocrine pathology practice. Endocr Pathol. 2022; 33:1–2.
Article3. Bai Y, Kakudo K, Jung CK. Updates in the pathologic classification of thyroid neoplasms: a review of the World Health Organization classification. Endocrinol Metab (Seoul). 2020; 35:696–715.
Article4. Kakudo K, Bychkov A, Bai Y, Li Y, Liu Z, Jung CK. The new 4th edition World Health Organization classification for thyroid tumors, Asian perspectives. Pathol Int. 2018; 68:641–64.
Article5. WHO Classification of Tumours Editorial Board. Endocrine and neuroendocrine tumours [Internet]. Lyon: International Agency for Research on Cancer;2022. [cited 2022 Sep 19]. Available from: https://tumourclassification.iarc.who.int/chapters/36.6. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022; 33:27–63.
Article7. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159:676–90.8. Yoo SK, Lee S, Kim SJ, Jee HG, Kim BA, Cho H, et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016; 12:e1006239.
Article9. Asa SL, Mete O. Oncocytic change in thyroid pathology. Front Endocrinol (Lausanne). 2021; 12:678119.
Article10. Bruford EA, Antonescu CR, Carroll AJ, Chinnaiyan A, Cree IA, Cross NC, et al. HUGO Gene Nomenclature Committee (HGNC) recommendations for the designation of gene fusions. Leukemia. 2021; 35:3040–3.
Article11. Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010; 23:1191–200.
Article12. Kim TH, Lee M, Kwon AY, Choe JH, Kim JH, Kim JS, et al. Molecular genotyping of the non-invasive encapsulated follicular variant of papillary thyroid carcinoma. Histopathology. 2018; 72:648–61.
Article13. Lee SR, Jung CK, Kim TE, Bae JS, Jung SL, Choi YJ, et al. Molecular genotyping of follicular variant of papillary thyroid carcinoma correlates with diagnostic category of fineneedle aspiration cytology: values of RAS mutation testing. Thyroid. 2013; 23:1416–22.
Article14. Jung CK, Kim Y, Jeon S, Jo K, Lee S, Bae JS. Clinical utility of EZH1 mutations in the diagnosis of follicular-patterned thyroid tumors. Hum Pathol. 2018; 81:9–17.
Article15. Jung CK, Bychkov A, Song DE, Kim JH, Zhu Y, Liu Z, et al. Molecular correlates and nuclear features of encapsulated follicular-patterned thyroid neoplasms. Endocrinol Metab (Seoul). 2021; 36:123–33.
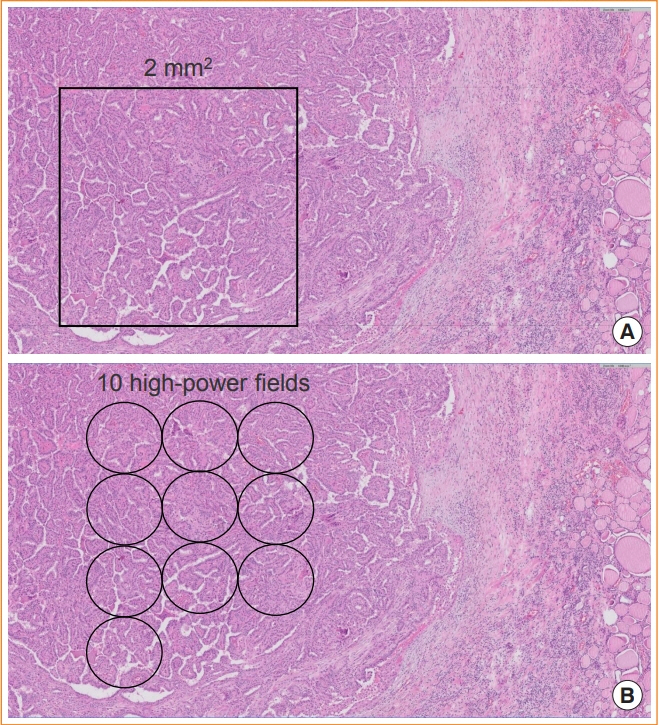
Article16. Cree IA, Tan PH, Travis WD, Wesseling P, Yagi Y, White VA, et al. Counting mitoses: SI(ze) matters! Mod Pathol. 2021; 34:1651–7.
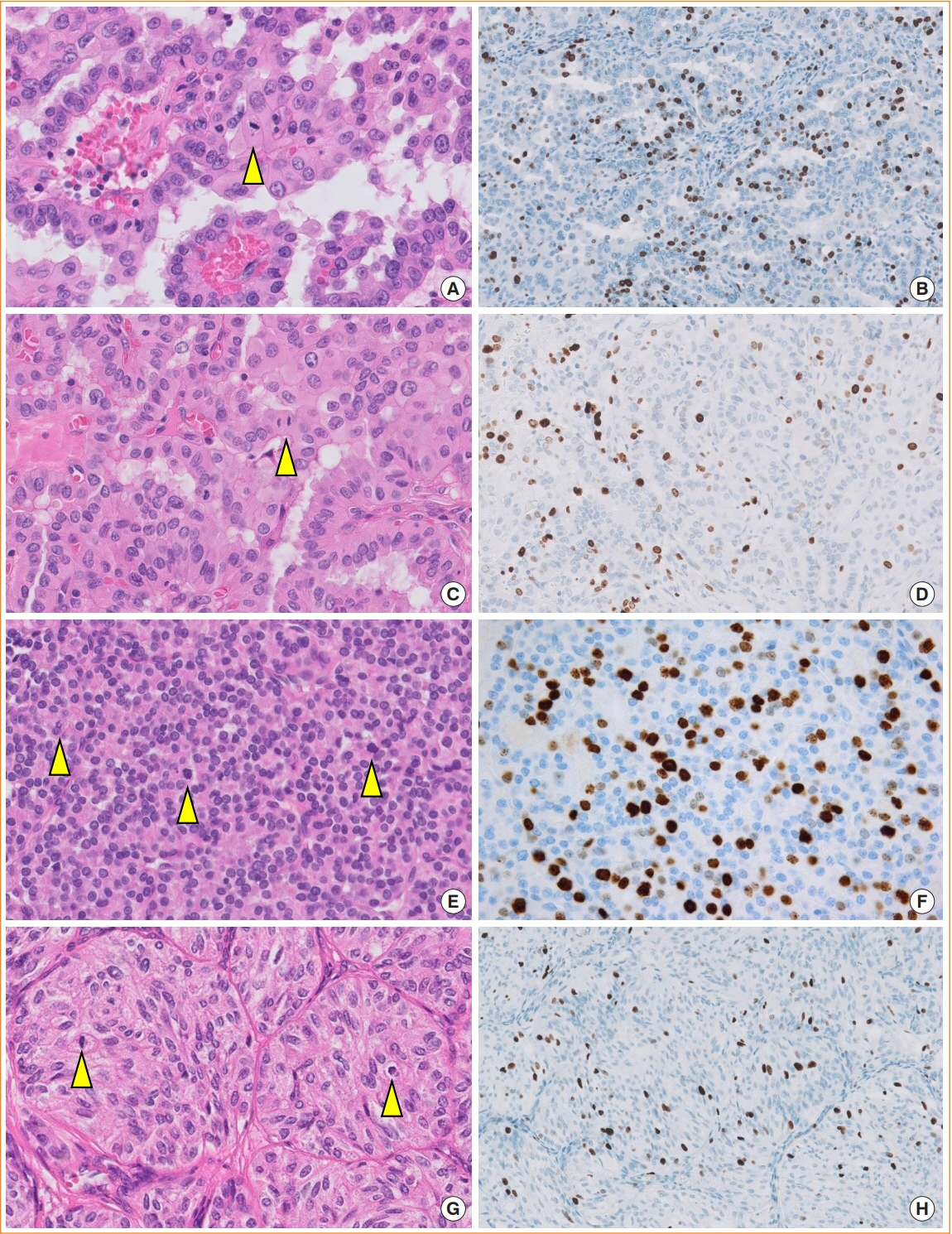
Article17. Hellgren LS, Stenman A, Paulsson JO, Hoog A, Larsson C, Zedenius J, et al. Prognostic utility of the Ki-67 labeling index in follicular thyroid tumors: a 20-year experience from a tertiary thyroid center. Endocr Pathol. 2022; 33:231–42.
Article18. Cree IA. From counting mitoses to Ki67 assessment: technical pitfalls in the new WHO classification of endocrine and neuroendocrine tumors. Endocr Pathol. 2022; 33:3–5.
Article19. Xu B, Fuchs TL, Ahmadi S, Alghamdi M, Alzumaili B, Bani MA, et al. International medullary thyroid carcinoma grading system: a validated grading system for medullary thyroid carcinoma. J Clin Oncol. 2022; 40:96–104.
Article20. Calebiro D, Grassi ES, Eszlinger M, Ronchi CL, Godbole A, Bathon K, et al. Recurrent EZH1 mutations are a second hit in autonomous thyroid adenomas. J Clin Invest. 2016; 126:3383–8.
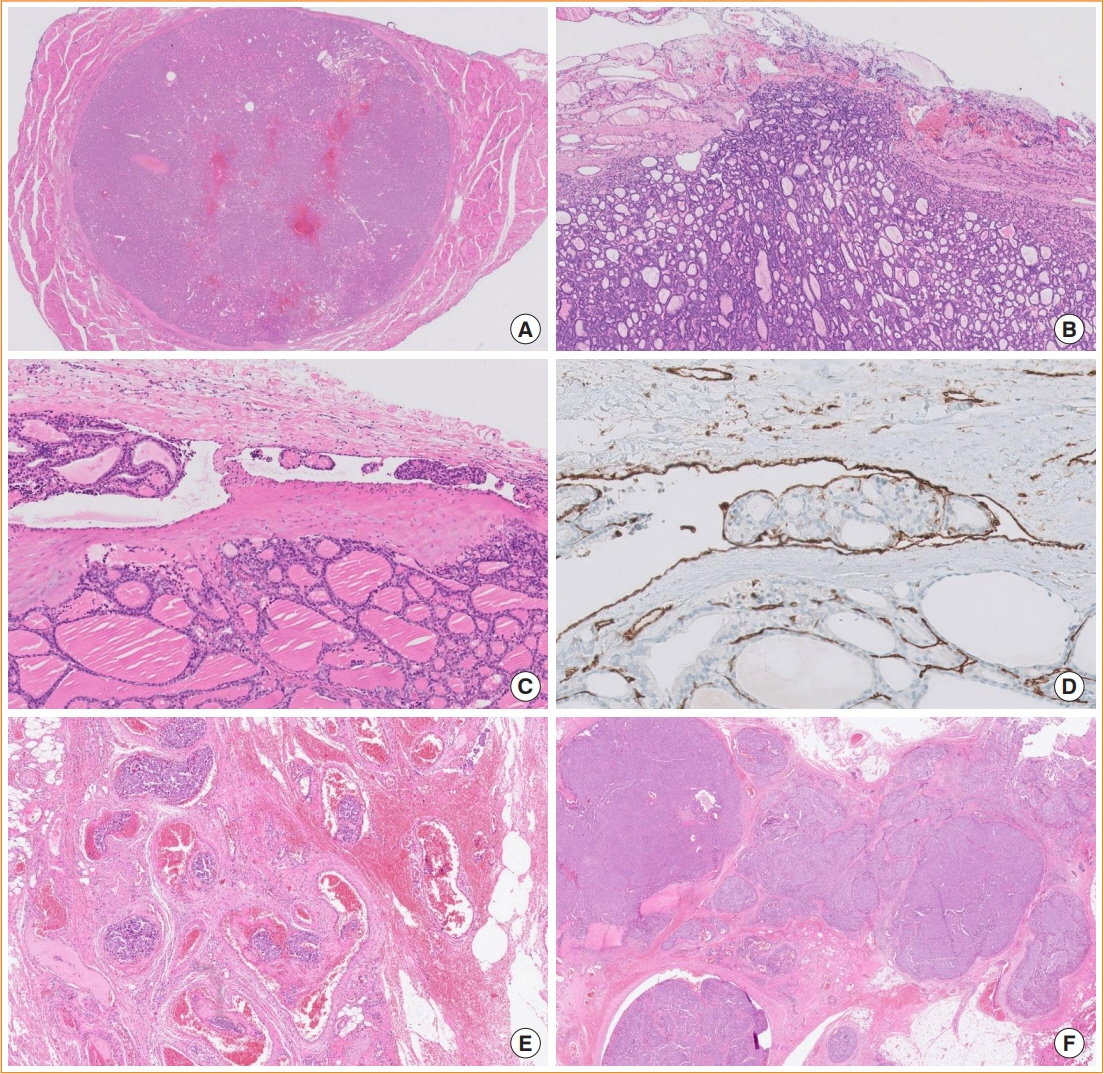
Article21. Cho U, Mete O, Kim MH, Bae JS, Jung CK. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol. 2017; 30:810–25.
Article22. Nikiforov YE, Baloch ZW, Hodak SP, Giordano TJ, Lloyd RV, Seethala RR, et al. Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillarylike nuclear features. JAMA Oncol. 2018; 4:1125–6.
Article23. Xu B, Serrette R, Tuttle RM, Alzumaili B, Ganly I, Katabi N, et al. How many papillae in conventional papillary carcinoma?: a clinical evidence-based pathology study of 235 unifocal encapsulated papillary thyroid carcinomas, with emphasis on the diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2019; 29:1792–803.
Article24. Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016; 2:1023–9.
Article25. Xu B, Reznik E, Tuttle RM, Knauf J, Fagin JA, Katabi N, et al. Outcome and molecular characteristics of non-invasive encapsulated follicular variant of papillary thyroid carcinoma with oncocytic features. Endocrine. 2019; 64:97–108.
Article26. Xu B, Farhat N, Barletta JA, Hung YP, Biase D, Casadei GP, et al. Should subcentimeter non-invasive encapsulated, follicular variant of papillary thyroid carcinoma be included in the noninvasive follicular thyroid neoplasm with papillary-like nuclear features category? Endocrine. 2018; 59:143–50.
Article27. Ito Y, Hirokawa M, Miyauchi A, Higashiyama T, Kihara M, Miya A. Prognostic significance of the proportion of tall cell components in papillary thyroid carcinoma. World J Surg. 2017; 41:742–7.
Article28. Wong KS, Chen TY, Higgins SE, Howitt BE, Lorch JH, Alexander EK, et al. A potential diagnostic pitfall for hobnail variant of papillary thyroid carcinoma. Histopathology. 2020; 76:707–13.
Article29. Cameselle-Teijeiro JM, Eloy C, Sobrinho-Simoes M. Pitfalls in challenging thyroid tumors: emphasis on differential diagnosis and ancillary biomarkers. Endocr Pathol. 2020; 31:197–217.
Article30. Cho J, Shin JH, Hahn SY, Oh YL. Columnar cell variant of papillary thyroid carcinoma: ultrasonographic and clinical differentiation between the indolent and aggressive types. Korean J Radiol. 2018; 19:1000–5.
Article31. Chen JH, Faquin WC, Lloyd RV, Nose V. Clinicopathological and molecular characterization of nine cases of columnar cell variant of papillary thyroid carcinoma. Mod Pathol. 2011; 24:739–49.
Article32. Lam AK. Squamous cell carcinoma of thyroid: a unique type of cancer in World Health Organization Classification. Endocr Relat Cancer. 2020; 27:R177–92.
Article33. Lai WA, Hang JF, Liu CY, Bai Y, Liu Z, Gu H, et al. PAX8 expression in anaplastic thyroid carcinoma is less than those reported in early studies: a multi-institutional study of 182 cases using the monoclonal antibody MRQ-50. Virchows Arch. 2020; 476:431–7.
Article34. Chambers MA, Sadow PM, Kerr DA. Squamous differentiation in the thyroid: metaplasia, neoplasia, or bystander? Int J Surg Pathol. 2022; 30:385–92.
Article35. Ito Y, Miyauchi A, Nakamura Y, Miya A, Kobayashi K, Kakudo K. Clinicopathologic significance of intrathyroidal epithelial thymoma/carcinoma showing thymus-like differentiation: a collaborative study with Member Institutes of The Japanese Society of Thyroid Surgery. Am J Clin Pathol. 2007; 127:230–6.
Article36. Saliba M, Mohanty AS, Ho AL, Drilon A, Dogan S. Secretory carcinoma of the thyroid in a 49-year-old man treated with larotrectinib: protracted clinical course of disease despite the high-grade histologic features. Head Neck Pathol. 2022; 16:612–20.
Article37. Tirado Y, Williams MD, Hanna EY, Kaye FJ, Batsakis JG, El-Naggar AK. CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin’s tumors: implications for histogenesis and biologic behavior. Genes Chromosomes Cancer. 2007; 46:708–15.
Article38. Shah AA, La Fortune K, Miller C, Mills SE, Baloch Z, LiVolsi V, et al. Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia: a clinicopathologic and molecular analysis of a distinct entity. Mod Pathol. 2017; 30:329–39.
Article39. Agaimy A, Togel L, Stoehr R, Meidenbauer N, Semrau S, Hartmann A, et al. NSD3-NUTM1-rearranged carcinoma of the median neck/thyroid bed developing after recent thyroidectomy for sclerosing mucoepidermoid carcinoma with eosinophilia: report of an extraordinary case. Virchows Arch. 2021; 479:1095–9.
Article40. Wiles AB, Kraft AO, Mueller SM, Powers CN. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: case report of a rare lesion with novel genetic mutation. Diagn Cytopathol. 2019; 47:589–93.
Article41. Hunt JL, LiVolsi VA, Barnes EL. p63 expression in sclerosing mucoepidermoid carcinomas with eosinophilia arising in the thyroid. Mod Pathol. 2004; 17:526–9.
Article42. Werner RG, Langlouis-Gau H, Walz F, Allgaier H, Hoffmann H. Validation of biotechnological production processes. Arzneimittelforschung. 1988; 38:855–62.43. Noor M, Russell DK, Israel AK, Lott Limbach A. Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia in conjunction with parotid basal cell adenoma: cytologic, histologic, and molecular features. Diagn Cytopathol. 2021; 49:E262–8.
Article44. Cameselle-Teijeiro JM, Peteiro-Gonzalez D, Caneiro-Gomez J, Sanchez-Ares M, Abdulkader I, Eloy C, et al. Cribriform-morular variant of thyroid carcinoma: a neoplasm with distinctive phenotype associated with the activation of the WNT/β-catenin pathway. Mod Pathol. 2018; 31:1168–79.
Article45. Boyraz B, Sadow PM, Asa SL, Dias-Santagata D, Nose V, Mete O. Cribriform-morular thyroid carcinoma is a distinct thyroid malignancy of uncertain cytogenesis. Endocr Pathol. 2021; 32:327–35.
Article46. Nose V. Familial thyroid cancer: a review. Mod Pathol. 2011; 24 Suppl 2:S19–33.
Article47. Guilmette J, Nose V. Hereditary and familial thyroid tumours. Histopathology. 2018; 72:70–81.
Article48. Rooper LM, Bynum JP, Miller KP, Lin MT, Gagan J, Thompson LD, et al. Recurrent DICER1 hotspot mutations in malignant thyroid gland teratomas: molecular characterization and proposal for a separate classification. Am J Surg Pathol. 2020; 44:826–33.49. Agaimy A, Witkowski L, Stoehr R, Cuenca JC, GonzalezMuller CA, Brutting A, et al. Malignant teratoid tumor of the thyroid gland: an aggressive primitive multiphenotypic malignancy showing organotypical elements and frequent DICER1 alterations-is the term “thyroblastoma” more appropriate? Virchows Arch. 2020; 477:787–98.
Article50. Yang J, Sarita-Reyes C, Kindelberger D, Zhao Q. A rare malignant thyroid carcinosarcoma with aggressive behavior and DICER1 gene mutation: a case report with literature review. Thyroid Res. 2018; 11:11.
Article51. Wong KS, Dong F, Telatar M, Lorch JH, Alexander EK, Marqusee E, et al. Papillary thyroid carcinoma with highgrade features versus poorly differentiated thyroid carcinoma: an analysis of clinicopathologic and molecular features and outcome. Thyroid. 2021; 31:933–40.
Article52. Xu B, David J, Dogan S, Landa I, Katabi N, Saliba M, et al. Primary high-grade non-anaplastic thyroid carcinoma: a retrospective study of 364 cases. Histopathology. 2022; 80:322–37.
Article53. Rivera M, Ghossein RA, Schoder H, Gomez D, Larson SM, Tuttle RM. Histopathologic characterization of radioactive iodine-refractory fluorodeoxyglucose-positron emission tomography-positive thyroid carcinoma. Cancer. 2008; 113:48–56.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Updates in the Pathologic Classification of Thyroid Neoplasms: A Review of the World Health Organization Classification
- Understanding Neoplasm of Uncertain or Unknown Behavior of the Thyroid in Korean Clinical Practice
- Recent Update of Pathology of the Pancreatic Neuroendocrine Tumor
- Major Changes to the 2017 Revision of the World Health Organization Classification of Myeloproliferative Neoplasms
- Borderline Thyroid Tumors: a Surgeon's Perspectives