Biomarker dynamics during infliximab salvage for acute severe ulcerative colitis: C-reactive protein (CRP)-lymphocyte ratio and CRP-albumin ratio are useful in predicting colectomy
- Affiliations
-
- 1Department of Gastroenterology, Eastern Health, Melbourne, Australia
- 2Eastern Health Clinical School, Faculty of Medicine, Nursing and Health Science, Monash University, Melbourne, Australia
- KMID: 2525077
- DOI: http://doi.org/10.5217/ir.2020.00146
Abstract
- Background/Aims
The residual risk of colectomy after infliximab salvage in steroid-refractory acute severe ulcerative colitis (ASUC) is required to inform the need for subsequent maintenance biologic therapy. The aim of this study was to determine the dynamic response of common serum biomarkers to infliximab salvage and assess their utility in predicting subsequent colectomy.
Methods
A retrospective single-center cohort study was conducted on all patients who received infliximab salvage for steroid-refractory ASUC between January 1, 2010, and July 31, 2019. Biomarkers were assessed on admission and days 1 and 3 post infliximab, and included C-reactive protein (CRP)-albumin-ratio (CAR), CRP-lymphocyte-ratio (CLR), platelet-lymphocyte-ratio (PLR) and neutrophil-lymphocyte-ratio (NLR).
Results
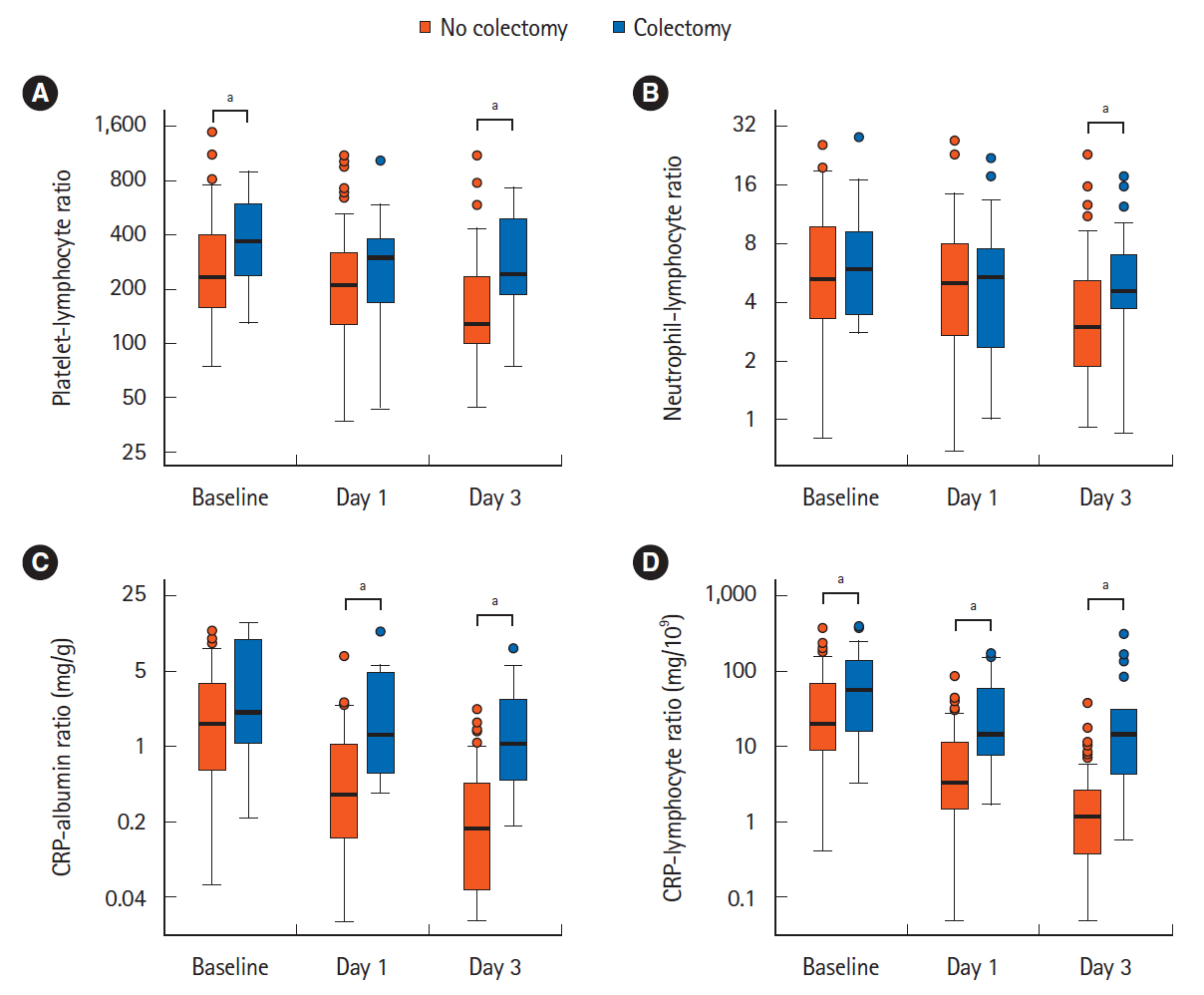
Of 94 patients (median age, 35 years; 67% of male), 20% required colectomy at 12 months. Biomarkers on day 3 post-infliximab best differentiated nonresponders, who had higher CRP, lower albumin and lower lymphocyte count (each P< 0.05). Day 3 predictive performance (area under the curve) for 12-month colectomy was best for CAR (0.871) and CLR (0.874), which were similar to Lindgren (0.829; P> 0.05) but superior to Mayo (0.726), partial Mayo (0.719), PLR (0.719), Ho index (0.714), NLR (0.675), Travis score (0.657) and endoscopic Mayo (0.609) (each P< 0.05). A day 3 CAR cutoff of 0.47 mg/g had 79% sensitivity, 80% specificity, 94% negative predictive value (NPV) to predict colectomy; while a day 3 CLR cutoff of 6.0 mg/109 had 84% sensitivity, 84% specificity, 96% NPV.
Conclusions
CAR and CLR measured on day 3 post infliximab salvage for steroid-refractory ASUC represent simple and routinely performed biomarkers that appear to be strong predictors of colectomy. Prospective studies are required to confirm the utility of these predictive scores.
Figure
Cited by 3 articles
-
Clinical outcomes and predictors of response for adalimumab in patients with moderately to severely active ulcerative colitis: a KASID prospective multicenter cohort study
Seung Yong Shin, Soo Jung Park, Young Kim, Jong Pil Im, Hyo Jong Kim, Kang-Moon Lee, Ji Won Kim, Sung-Ae Jung, Jun Lee, Sang-Bum Kang, Sung Jae Shin, Eun Sun Kim, You Sun Kim, Tae Oh Kim, Hyun-Soo Kim, Dong Il Park, Hyung Kil Kim, Eun Soo Kim, Young-Ho Kim, Do Hyun Kim, Dennis Teng, Jong-Hwa Kim, Wonyong Kim, Chang Hwan Choi
Intest Res. 2022;20(3):350-360. doi: 10.5217/ir.2021.00049.Ischemia-modified albumin: a novel blood marker of endoscopic mucosal healing in inflammatory bowel disease
Seung Bum Lee, Hyun-Ki Kim, Sang Hyuk Park, Ji-Hun Lim, Sang Hyoung Park
Intest Res. 2024;22(1):75-81. doi: 10.5217/ir.2023.00065.Which biomarkers best reflect the degree of inflammation in Crohn’s disease?
Jihye Park
Intest Res. 2024;22(1):1-2. doi: 10.5217/ir.2023.00161.
Reference
-
1. Seah D, De Cruz P. Review article: the practical management of acute severe ulcerative colitis. Aliment Pharmacol Ther. 2016; 43:482–513.
Article2. Jackson BD, Con D, De Cruz P. Design considerations for an eHealth decision support tool in inflammatory bowel disease self-management. Intern Med J. 2018; 48:674–681.
Article3. Fukuda T, Naganuma M, Kanai T. Current new challenges in the management of ulcerative colitis. Intest Res. 2019; 17:36–44.
Article4. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955; 2:1041–1048.5. Levy LC, Coburn ES, Choi S, Holubar SD. The management of the hospitalized ulcerative colitis patient: the medical-surgical conundrum. Curr Opin Gastroenterol. 2020; 36:265–276.
Article6. Verdon C, Bessissow T, Lakatos PL. Management of acute severe colitis in the era of biologicals and small molecules. J Clin Med. 2019; 8:2169.
Article7. Oh SJ, Shin GY, Soh H, et al. Long-term outcomes of infliximab in a real-world multicenter cohort of patients with acute severe ulcerative colitis. Intest Res. 2021; 19:323–331.
Article8. Aratari A, Papi C, Clemente V, et al. Colectomy rate in acute severe ulcerative colitis in the infliximab era. Dig Liver Dis. 2008; 40:821–826.
Article9. Gustavsson A, Järnerot G, Hertervig E, et al. Clinical trial: colectomy after rescue therapy in ulcerative colitis: 3-year follow-up of the Swedish-Danish controlled infliximab study. Aliment Pharmacol Ther. 2010; 32:984–989.
Article10. Laharie D, Bourreille A, Branche J, et al. Long-term outcome of patients with steroid-refractory acute severe UC treated with ciclosporin or infliximab. Gut. 2018; 67:237–243.
Article11. Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009; 44:431–440.
Article12. Bernardo S, Fernandes SR, Gonçalves AR, et al. Predicting the course of disease in hospitalized patients with acute severe ulcerative colitis. Inflamm Bowel Dis. 2019; 25:541–546.
Article13. Denson LA, Curran M, McGovern DP, et al. Challenges in IBD research: precision medicine. Inflamm Bowel Dis. 2019; 25(Suppl 2):S31–S39.
Article14. Li Wai Suen CF, Choy MC, De Cruz P. Letter: infliximab induction regimens in steroid-refractory acute severe colitis-a propensity score analysis. Aliment Pharmacol Ther. 2020; 51:665–666.
Article15. Choy MC, Seah D, Gorelik A, et al. Predicting response after infliximab salvage in acute severe ulcerative colitis. J Gastroenterol Hepatol. 2018; 33:1347–1352.
Article16. Battat R, Hemperly A, Truong S, et al. Baseline clearance of infliximab is associated with requirement for colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2021; 19:511–518.
Article17. Honig G, Heller C, Hurtado-Lorenzo A. Defining the path forward for biomarkers to address unmet needs in inflammatory bowel diseases. Inflamm Bowel Dis. 2020; 26:1451–1462.
Article18. Porter AC, Aubrecht J, Birch C, et al. Biomarkers of Crohn’s disease to support the development of new therapeutic interventions. Inflamm Bowel Dis. 2020; 26:1498–1508.
Article19. Bertani L, Rossari F, Barberio B, et al. Novel prognostic biomarkers of mucosal healing in ulcerative colitis patients treated with anti-TNF: neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. Inflamm Bowel Dis. 2020; 26:1579–1587.
Article20. Jeong Y, Jeon SR, Kim HG, et al. The role of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in ulcerative colitis. Intest Res. 2021; 19:62–70.
Article21. Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg. 2020; 272:342–351.
Article22. Ho GT, Mowat C, Goddard CJ, et al. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther. 2004; 19:1079–1087.
Article23. Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996; 38:905–910.
Article24. Lindgren SC, Flood LM, Kilander AF, Löfberg R, Persson TB, Sjödahl RI. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur J Gastroenterol Hepatol. 1998; 10:831–835.
Article25. Järnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005; 128:1805–1811.
Article26. Con D, Parthasarathy N, Bishara M, et al. Development of a simple, serum biomarker-based model predictive of the need for early biologic therapy in Crohn’s disease. J Crohns Colitis. 2021; 15:583–593.
Article27. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019; 381:1201–1214.
Article28. Sands BE, Peyrin-Biroulet L, Loftus EV Jr, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019; 381:1215–1226.
Article29. Deepak P, Alayo QA, Khatiwada A, et al. Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021; 19:1592–1601.
Article30. Hibi T, Kamae I, Pinton P, et al. Efficacy of biologic therapies for biologic-naïve Japanese patients with moderately to severely active ulcerative colitis: a network meta-analysis. Intest Res. 2021; 19:53–61.
Article31. Kotwani P, Terdiman J, Lewin S. Tofacitinib for rescue therapy in acute severe ulcerative colitis: a real-world experience. J Crohns Colitis. 2020; 14:1026–1028.
Article32. Weisshof R, Ollech JE, El Jurdi K, et al. Ciclosporin therapy after infliximab failure in hospitalized patients with acute severe colitis is effective and safe. J Crohns Colitis. 2019; 13:1105–1110.
Article33. Seah D, Choy MC, Gorelik A, et al. Examining maintenance care following infliximab salvage therapy for acute severe ulcerative colitis. J Gastroenterol Hepatol. 2018; 33:226–231.
Article34. Vasudevan A, Arachchi A, Scanlon C, Greenhalgh J, Van Langenberg DR. A comparison of long-term healthcare utilization and costs in patients with acute severe ulcerative colitis receiving infliximab versus early colectomy. Ther Adv Chronic Dis. 2019; 10:2040622319825595.35. Jackson B, Con D, Ma R, Gorelik A, Liew D, De Cruz P. Health care costs associated with Australian tertiary inflammatory bowel disease care. Scand J Gastroenterol. 2017; 52:851–856.
Article36. Soufleris K, Kafalis N, Charalampidis M, et al. P426 Lympocytosis in patients with inflammatory bowel disease treated with anti-TNFa agents: is it significant? J Crohns Colitis. 2019; 13(Suppl_1):S322.
Article37. Aeberli D, Seitz M, Jüni P, Villiger PM. Increase of peripheral CXCR3 positive T lymphocytes upon treatment of RA patients with TNF-alpha inhibitors. Rheumatology (Oxford). 2005; 44:172–175.
Article38. Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010; 48:297–308.
Article39. Syal G, Robbins L, Kashani A, et al. Hypoalbuminemia and bandemia predict failure of infliximab rescue therapy in acute severe ulcerative colitis. Dig Dis Sci. 2021; 66:199–205.
Article40. Beswick L, Rosella O, Rosella G, et al. Exploration of predictive biomarkers of early infliximab response in acute severe colitis: a prospective pilot study. J Crohns Colitis. 2018; 12:289–297.
Article41. Sebastian S, Myers S, Argyriou K, et al. Infliximab induction regimens in steroid-refractory acute severe colitis: a multicentre retrospective cohort study with propensity score analysis. Aliment Pharmacol Ther. 2019; 50:675–683.
Article42. Nalagatla N, Falloon K, Tran G, et al. Effect of accelerated infliximab induction on short- and long-term outcomes of acute severe ulcerative colitis: a retrospective multicenter study and meta-analysis. Clin Gastroenterol Hepatol. 2019; 17:502–509.
Article43. Con D, Jackson B, Gray K, De Cruz P. eHealth for inflammatory bowel disease self-management: the patient perspective. Scand J Gastroenterol. 2017; 52:973–980.44. Dubinsky MC. Reviewing treatments and outcomes in the evolving landscape of ulcerative colitis. Postgrad Med. 2017; 129:538–553.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- C-reactive protein/albumin ratio as prognostic score in oral squamous cell carcinoma
- Neutrophil-Lymphocyte Ratio as Inflammatory Marker for Delirium: An Exploratory Study
- High-sensitivity C-reactive protein/albumin ratio as a predictor of in-hospital mortality in older adults admitted to the emergency department
- Infliximab versus Cyclosporine Treatment for Severe Corticosteroid-Refractory Ulcerative Colitis: A Korean, Retrospective, Single Center Study
- Clinical significance of C-reactive protein-to-prealbumin ratio in predicting early recurrence in resectable pancreatic cancer