Cancer Res Treat.
2021 Jan;53(1):87-92. 10.4143/crt.2020.741.
Phase II Study of Pemetrexed as a Salvage Chemotherapy for Thymidylate Synthase–Low Squamous Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, The Catholic University of Korea Incheon St. Mary’s Hospital, Incheon, Korea
- 2Division of Hematology and Oncology, Department of Internal Medicine, National Cancer Center, Goyang, Korea
- 3Center for Lung Cancer, National Cancer Center, Goyang, Korea
- 4Department of Pathology, National Cancer Center, Goyang, Korea
- 5Department of Radiology, National Cancer Center, Goyang, Korea
- 6Office of Biostatistics Research, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, USA
- KMID: 2510650
- DOI: http://doi.org/10.4143/crt.2020.741
Abstract
- Purpose
Squamous cell carcinomas (SqCC) of the lung often express high levels of thymidylate synthase (TS), which is associated with primary resistance to pemetrexed. We explored the efficacy of pemetrexed in a selected population of patients with lung SqCC with low TS expression.
Materials and Methods
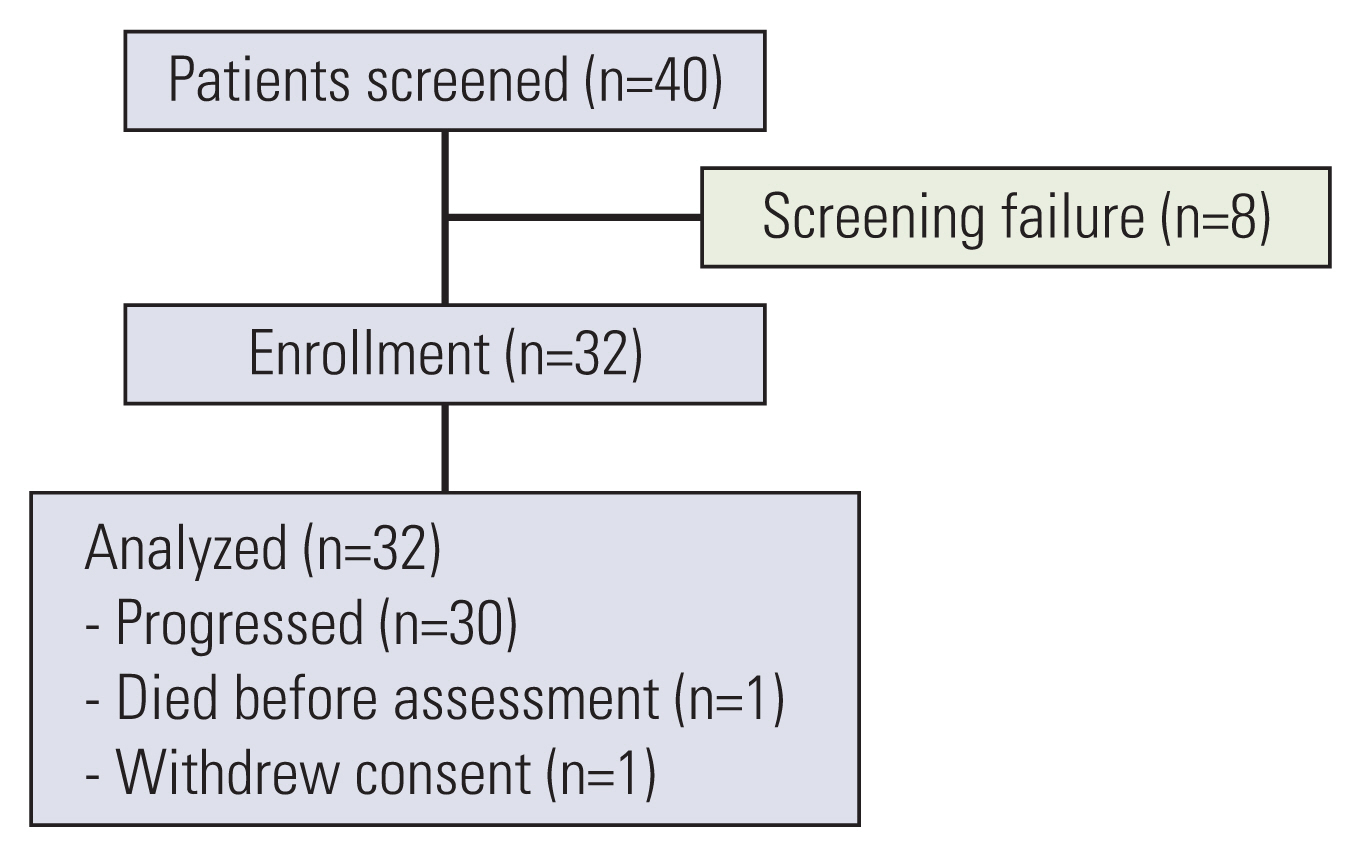
In this single-arm phase II trial, we enrolled 32 previously-treated patients with advanced lung SqCC exhibiting low immunohistochemical staining for TS (i.e., in 10% or less of tumor cells). The primary endpoint was 12-week progression-free survival (PFS) rate.
Results
Of 32 patients, eight patients (25%) had an Eastern Cooperative Oncology Group performance status of 2, and seven patients (22%) had previously received three or more lines of chemotherapy. The disease control rate from pemetrexed treatment was 30%, and no objective response was observed. The 12-week PFS rate was 24.5% (95% confidence interval [CI], 13.0 to 46.1). Median PFS was 1.3 months (95% CI, 1.3 to 2.7), and median overall survival was 11.8 months (95% CI, 8.1 to not applicable). Most of adverse events were grade 1 or 2.
Conclusion
Pemetrexed demonstrated modest activity as a salvage chemotherapy in patients with advanced lung SqCC with low TS expression, although its toxicity was generally manageable.
Figure
Reference
-
References
1. Travis WD. Lung cancer pathology: current concepts. Clin Chest Med. 2020; 41:67–85.2. Sogaard M, Thomsen RW, Bossen KS, Sorensen HT, Norgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013; 5:3–29.
Article3. Putila J, Guo NL. Combining COPD with clinical, pathological and demographic information refines prognosis and treatment response prediction of non-small cell lung cancer. PLoS One. 2014; 9:e100994.
Article4. Heist RS, Sequist LV, Engelman JA. Genetic changes in squamous cell lung cancer: a review. J Thorac Oncol. 2012; 7:924–33.
Article5. Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012; 489:519–25.6. Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017; 35:3924–33.
Article7. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. Network Engl J Med. 2018; 379:2040–51.
Article8. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019; 37:537–46.
Article9. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004; 22:1589–97.
Article10. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008; 26:3543–51.
Article11. Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009; 374:1432–40.
Article12. Scagliotti G, Hanna N, Fossella F, Sugarman K, Blatter J, Peterson P, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist. 2009; 14:253–63.
Article13. Scagliotti G, Brodowicz T, Shepherd FA, Zielinski C, Vansteenkiste J, Manegold C, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011; 6:64–70.
Article14. Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007; 6:404–17.
Article15. Ozasa H, Oguri T, Uemura T, Miyazaki M, Maeno K, Sato S, et al. Significance of thymidylate synthase for resistance to pemetrexed in lung cancer. Cancer Sci. 2010; 101:161–6.
Article16. Liu Y, Yin TJ, Zhou R, Zhou S, Fan L, Zhang RG. Expression of thymidylate synthase predicts clinical outcomes of pemetrexed-containing chemotherapy for non-small-cell lung cancer: a systemic review and meta-analysis. Cancer Chemother Pharmacol. 2013; 72:1125–32.
Article17. Sun JM, Ahn JS, Jung SH, Sun J, Ha SY, Han J, et al. Pemetrexed plus cisplatin versus gemcitabine plus cisplatin according to thymidylate synthase expression in nonsquamous non-small-cell lung cancer: a biomarker-stratified randomized phase II trial. J Clin Oncol. 2015; 33:2450–6.
Article18. Ceppi P, Volante M, Saviozzi S, Rapa I, Novello S, Cambieri A, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006; 107:1589–96.
Article19. Tanaka F, Wada H, Fukui Y, Fukushima M. Thymidylate synthase (TS) gene expression in primary lung cancer patients: a large-scale study in Japanese population. Ann Oncol. 2011; 22:1791–7.
Article20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article21. Niyikiza C, Baker SD, Seitz DE, Walling JM, Nelson K, Rusthoven JJ, et al. Homocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther. 2002; 1:545–52.22. Mandrekar SJ, Qi Y, Hillman SL, Allen Ziegler KL, Reuter NF, Rowland KM Jr, et al. Endpoints in phase II trials for advanced non-small cell lung cancer. J Thorac Oncol. 2010; 5:3–9.
Article23. Nicolson MC, Fennell DA, Ferry D, O’Byrne K, Shah R, Potter V, et al. Thymidylate synthase expression and outcome of patients receiving pemetrexed for advanced nonsquamous non-small-cell lung cancer in a prospective blinded assessment phase II clinical trial. J Thorac Oncol. 2013; 8:930–9.
Article24. Hou J, Lambers M, den Hamer B, den Bakker MA, Hoogsteden HC, Grosveld F, et al. Expression profiling-based subtyping identifies novel non-small cell lung cancer subgroups and implicates putative resistance to pemetrexed therapy. J Thorac Oncol. 2012; 7:105–14.
Article25. Liang J, Lu T, Chen Z, Zhan C, Wang Q. Mechanisms of resistance to pemetrexed in non-small cell lung cancer. Transl Lung Cancer Res. 2019; 8:1107–18.
Article26. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011; 364:2507–16.
Article27. Kopetz S, Desai J, Chan E, Hecht JR, O’Dwyer PJ, Maru D, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015; 33:4032–8.
Article28. Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012; 483:100–3.
Article29. Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010; 5:620–30.
Article30. Jamal-Hanjani M, Quezada SA, Larkin J, Swanton C. Translational implications of tumor heterogeneity. Clin Cancer Res. 2015; 21:1258–66.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Pemetrexed in Relapsed Non-Small Cell Lung Cancer and Thymidylate Synthase Expression
- The effect of Thymidylate Synthetase extression in stomach cancer tissues on the prognosis
- Effects of Lovastatin in Combination with 5-FU on Stomach Cancer Cells
- Phase II Study of S-1 Plus Either Irinotecan or Docetaxel for Non-small Cell Lung Cancer Patients Treated with More Than Three Lines of Treatment
- Interstitial Pneumonitis after Treatment with Pemetrexed for Non-small Cell Lung Cancer