Chonnam Med J.
2019 Jan;55(1):1-7. 10.4068/cmj.2019.55.1.1.
Apoptotic Cell-Mimetic Polymers for Anti-Inflammatory Therapy
- Affiliations
-
- 1International Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS), Tsukuba, Japan. EBARA.Mitsuhiro@nims.go.jp
- 2Graduate School of Pure and Applied Sciences, University of Tsukuba, Tsukuba, Japan.
- 3Graduate School of Industrial Science and Technology, Tokyo University of Science, Tokyo, Japan.
- KMID: 2432249
- DOI: http://doi.org/10.4068/cmj.2019.55.1.1
Abstract
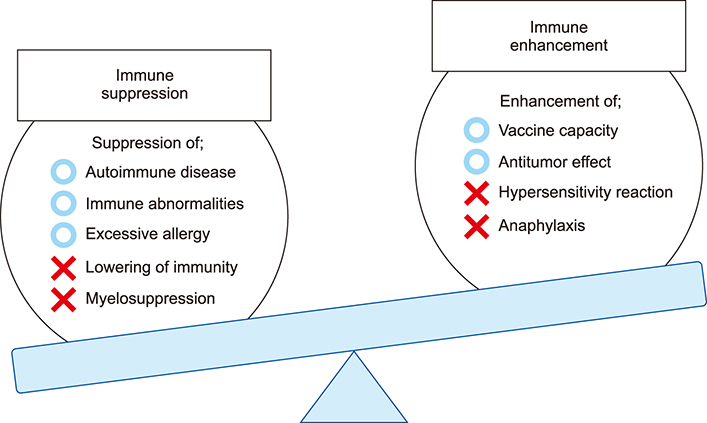
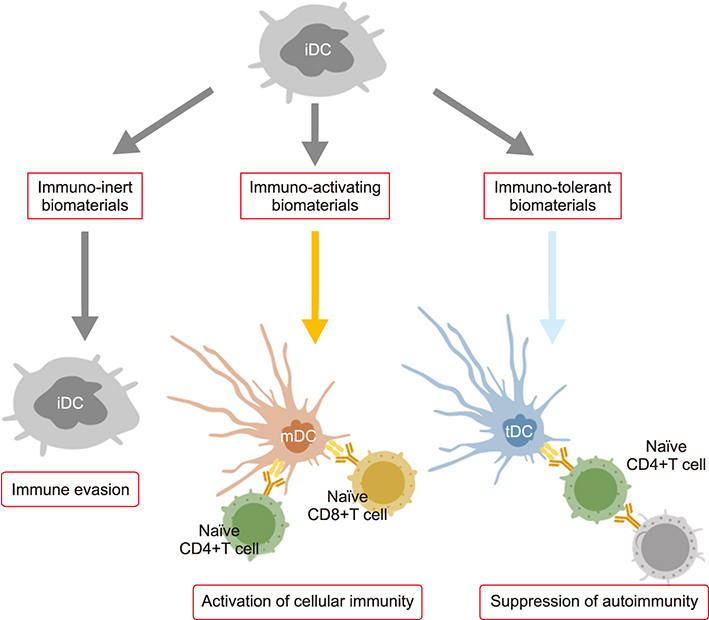
- The field of biomaterials has seen a strong rejuvenation due to the new potential to modulate immune system in our body. This special class of materials is called "immunomodulatory biomaterials". Generally, three fundamental strategies are followed in the design of immunomodulatory biomaterials: (1) immuno-inert biomaterials, (2) immuno-activating biomaterials, and (3) immuno-tolerant biomaterials. While many applications of immuno-inert biomaterials such as biocompatible medical implants have been already proposed in the past decades, the ability to engineer biological activity into synthetic materials greatly increases the number of their potential uses and improves their performance in more traditional applications. The major focus of researchers is now set on developing immuno-tolerant biomaterials for anti-inflammatory therapies. In this review, we therefore introduce recent developments of immuno-tolerant biomaterials. Especially we introduce an apoptotic cell membrane-inspired polymer and its post-inflammatory effects on immune cells in this article.
Keyword
MeSH Terms
Figure
Reference
-
1. Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004; 6:41–75.
Article2. Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nature. 2009; 462:426–432.
Article3. Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977; 252:3582–3586.
Article4. Uchida K, Hoshino Y, Tamura A, Yoshimoto K, Kojima S, Yamashita K, et al. Creation of a mixed poly(ethylene glycol) tethered-chain surface for preventing the nonspecific adsorption of proteins and peptides. Biointerphases. 2007; 2:126–130.
Article5. Alcantar NA, Aydil ES, Israelachvili JN. Polyethylene glycol-coated biocompatible surfaces. J Biomed Mater Res. 2000; 51:343–351.
Article6. Leckband D, Sheth S, Halperin A. Grafted poly(ethylene oxide) brushes as nonfouling surface coatings. J Biomater Sci Polym Ed. 1999; 10:1125–1147.
Article7. Ladd J, Zhang Z, Chen S, Hower JC, Jiang S. Zwitterionic polymers exhibiting high resistance to nonspecific protein adsorption from human serum and plasma. Biomacromolecules. 2008; 9:1357–1361.
Article8. Li A, Luehmann HP, Sun G, Samarajeewa S, Zou J, Zhang S, et al. Synthesis and in vivo pharmacokinetic evaluation of degradable shell cross-linked polymer nanoparticles with poly(carboxybetaine) versus poly(ethylene glycol) surface-grafted coatings. ACS Nano. 2012; 6:8970–8982.
Article9. Zheng L, Sundaram HS, Wei Z, Li C, Yuan Z. Applications of zwitterionic polymers. React Func Polym. 2017; 118:51–61.
Article10. Ishihara K, Aragaki R, Ueda T, Watenabe A, Nakabayashi N. Reduced thrombogenicity of polymers having phospholipid polar groups. J Biomed Mater Res. 1990; 24:1069–1077.
Article11. Goda T, Ishihara K, Miyahara Y. Critical update on 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer science. J Appl Polym Sci. 2015; 132:41766.
Article12. Mu M, Konno T, Ishihara K. Spontaneous hydrogel formation through hydrophobic interactions in an ABA-type block copolymer composed of poly(2-methacryloyloxyethyl phosphorylcholine) and poly(n-butyl methacrylate) segments. MRS Advances. 2018; 3:1691–1696.
Article13. Nilsson JS, Broos S, Akagi T, Akashi M, Hermansson A, Cayé-Thomasen P, et al. Amphiphilic γ-PGA nanoparticles administered on rat middle ear mucosa produce adjuvant-like immunostimulation in vivo. Acta Otolaryngol. 2014; 134:1034–1041.
Article14. Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005; 57:391–410.
Article15. Benoit MA, Baras B, Gillard J. Preparation and characterization of protein-loaded poly(epsilon-caprolactone) microparticles for oral vaccine delivery. Int J Pharm. 1999; 184:73–84.
Article16. Kwon YJ, James E, Shastri N, Fréchet JM. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on pH-responsive microparticles. Proc Natl Acad Sci U S A. 2005; 102:18264–18268.
Article17. Murthy N, Robichaud JR, Tirrell DA, Stayton PS, Hoffman AS. The design and synthesis of polymers for eukaryotic membrane disruption. J Control Release. 1999; 61:137–143.
Article18. Kaneda Y, Yamamoto S, Nakajima T. Development of HVJ envelope vector and its application to gene therapy. Adv Genet. 2005; 53:307–332.
Article19. Kurooka M, Kaneda Y. Inactivated Sendai virus particles eradicate tumors by inducing immune responses through blocking regulatory T cells. Cancer Res. 2007; 67:227–236.
Article20. Fisher KD, Stallwood Y, Green NK, Ulbrich K, Mautner V, Seymour LW. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001; 8:341–348.
Article21. Okada T, Uto K, Sasai M, Lee CM, Ebara M, Aoyagi T. Nano-decoration of the Hemagglutinating Virus of Japan envelope (HVJ-E) using a layer-by-layer assembly technique. Langmuir. 2013; 29:7384–7392.
Article22. Okada T, Uto K, Aoyagi T, Ebara M. A biomimetic approach to hormone resistant prostate cancer cell isolation using inactivated Sendai virus (HVJ-E). Biomater Sci. 2016; 4:96–103.
Article23. Phillips B, Nylander K, Harnaha J, Machen J, Lakomy R, Styche A, et al. A microsphere-based vaccine prevents and reverses new-onset autoimmune diabetes. Diabetes. 2008; 57:1544–1555.
Article24. Lewis JS, Zaveri TD, Crooks CP 2nd, Keselowsky BG. Micropar-particle surface modifications targeting dendritic cells for non-activating applications. Biomaterials. 2012; 33:7221–7232.
Article25. Yoshitomi T, Yamaguchi Y, Kikuchi A, Nagasaki Y. Creation of a blood-compatible surface: a novel strategy for suppressing blood activation and coagulation using a nitroxide radical-containing polymer with reactive oxygen species scavenging activity. Acta Biomater. 2012; 8:1323–1329.
Article26. Boonruamkaew P, Chonpathompikunlert P, Vong LB, Sakaue S, Tomidokoro Y, Ishii K, et al. Chronic treatment with a smart antioxidative nanoparticle for inhibition of amyloid plaque propagation in Tg2576 mouse model of Alzheimer's disease. Sci Rep. 2017; 7:3785.
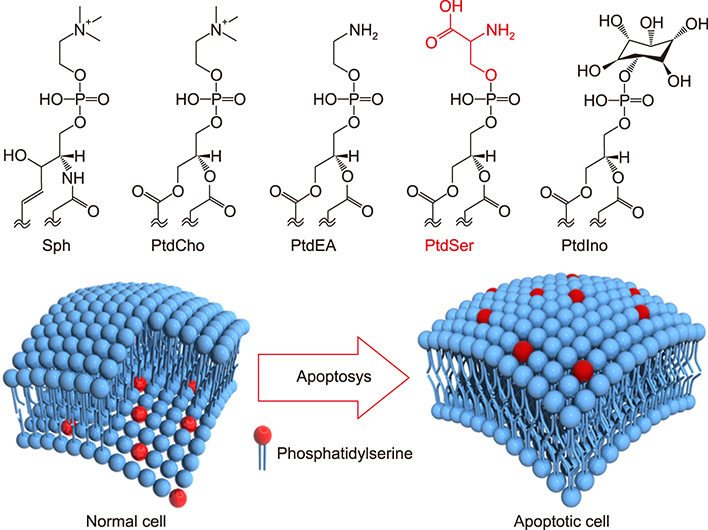
Article27. Verkleij AJ, Post JA. Membrane phospholipid asymmetry and signal transduction. J Membr Biol. 2000; 178:1–10.
Article28. Somersan S, Bhardwaj N. Tethering and tickling: a new role for the phosphatidylserine receptor. J Cell Biol. 2001; 155:501–504.29. Gaipl US, Beyer TD, Baumann I, Voll RE, Stach CM, Heyder P, et al. Exposure of anionic phospholipids serves as anti-inflammatory and immunosuppressive signal--implications for antiphospholipid syndrome and systemic lupus erythematosus. Immunobiology. 2003; 207:73–81.
Article30. Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997; 390:350–351.
Article31. Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J, Cohen S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci U S A. 2011; 108:1827–1832.
Article32. Hashioka S, Han YH, Fujii S, Kato T, Monji A, Utsumi H, et al. Phosphatidylserine and phosphatidylcholine-containing liposomes inhibit amyloid beta and interferon-gamma-induced microglial activation. Free Radic Biol Med. 2007; 42:945–954.
Article33. Wu Z, Ma HM, Kukita T, Nakanishi Y, Nakanishi H. Phosphatidylserine-containing liposomes inhibit the differentiation of osteoclasts and trabecular bone loss. J Immunol. 2010; 184:3191–3201.
Article34. Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002; 109:41–50.
Article35. Thum T, Bauersachs J, Poole-Wilson PA, Volk HD, Anker SD. The dying stem cell hypothesis: immune modulation as a novel mechanism for progenitor cell therapy in cardiac muscle. J Am Coll Cardiol. 2005; 46:1799–1802.36. Ren Y, Xie Y, Jiang G, Fan J, Yeung J, Li W, et al. Apoptotic cells protect mice against lipopolysaccharide-induced shock. J Immunol. 2008; 180:4978–4985.
Article37. Nolan Y, Martin D, Campbell VA, Lynch MA. Evidence of a protective effect of phosphatidylserine-containing liposomes on lipopolysaccharide-induced impairment of long-term potentiation in the rat hippocampus. J Neuroimmunol. 2004; 151:12–23.
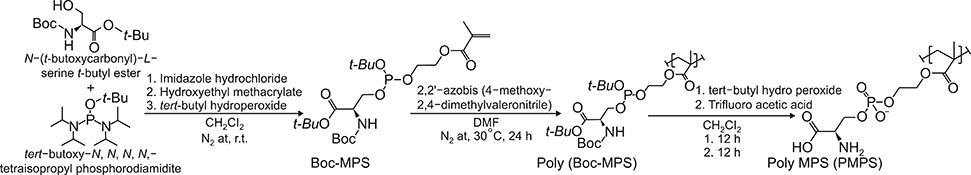
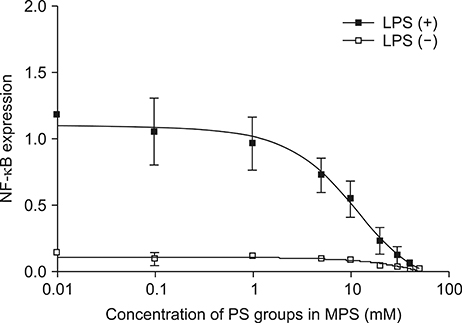
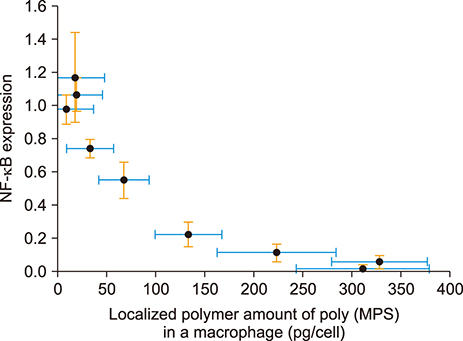
Article38. Nakagawa Y, Saitou A, Aoyagi T, Naito M, Ebara M. Apoptotic cell membrane-inspired polymer for immunosuppression. ACS Macro Lett. 2017; 6:1020–1024.
Article39. Nakagawa Y, Saitou A, Aoyagi T, Ebara M. Rational design of anti-inflammatory polymers inspired by apoptotic cell death using phosphoramidite chemistry. Polymer. 2018; 134:85–93.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory and anti-apoptotic effects of paricalcitol in lipopolysaccharide-induced renal proximal tubular cell injury
- Anti-HER2/neu Peptide Producing Vector System for Biologic Therapy - Is It Possible to Mass-produce the Peptide?
- Elm tree bark extract inhibits HepG2 hepatic cancer cell growth via pro-apoptotic activity
- Unlocking the Therapeutic Potential of BCL-2 Associated Protein Family: Exploring BCL-2 Inhibitors in Cancer Therapy
- Polymeric Gene Carriers and Their Applications to Diabetes Gene Therapy