Lab Anim Res.
2018 Mar;34(1):30-36. 10.5625/lar.2018.34.1.30.
The effect of near-infrared fluorescence conjugation on the anti-cancer potential of cetuximab
- Affiliations
-
- 1College of Veterinary Medicine and Research Institute of Veterinary Medicine, Chungbuk National University, Cheongju, Korea. beomjun@cbu.ac.kr
- 2Laboratory Animal Center, Osong Medical Innovation Foundation, Cheongju, Korea.
- 3College of Veterinary Medicine and Institute of Animal Medicine, Gyeongsang National University, Jinju, Korea. hujang@gnu.ac.kr
- KMID: 2430883
- DOI: http://doi.org/10.5625/lar.2018.34.1.30
Abstract
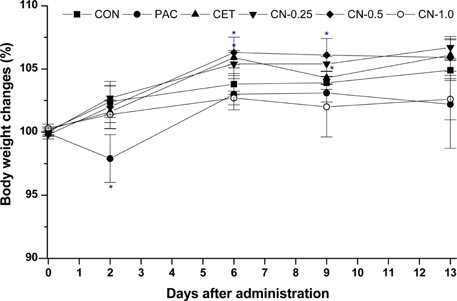
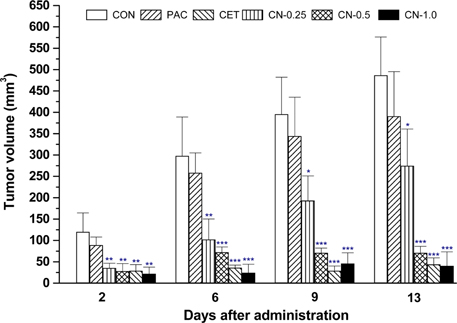
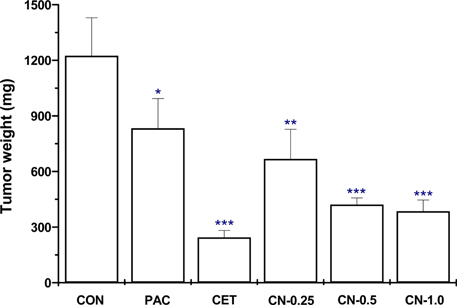
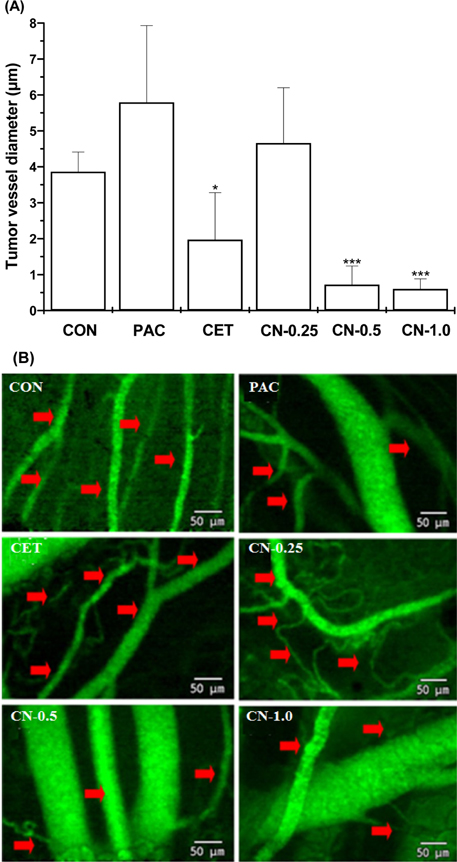
- This study investigated the anti-cancer potential of a near-infrared fluorescence (NIRF) molecule conjugated with Cetuximab (Cetuximab-NIRF) in six-week-old female BALB/c athymic (nu+/nu+) nude mice. A431 cells were cultured and injected into the animals to induce solid tumors. Paclitaxel (30 mg/kg body weight (BW)), Cetuximab (1 mg/kg BW), and Cetuximab-NIRF (0.25, 0.5 and 1.0 mg/kg BW) were intraperitoneally injected twice a week into the A431 cell xenografts of the nude mice. Changes in BW, tumor volume and weight, fat and lean mass, and diameter of the peri-tumoral blood vessel were determined after two weeks. Tumor volumes and weights were significantly decreased in the Cetuximab-NIRF (1 mg/kg BW) group compared with the control group (P < 0.001). Lean mass and total body water content were also conspicuously reduced in the Cetuximab-NIRF (1 mg/kg BW) group compared with the vehicle control group. Peri-tumoral blood vessel diameters were very thin in the Cetuximab-NIRF groups compared with those of the paclitaxel group. These results indicate that the conjugation of Cetuximab with NIRF does not affect the anti-cancer potential of Cetuximab and NIRF can be used for molecular imaging in cancer treatments.
MeSH Terms
Figure
Reference
-
1. Arteaga CL. EGF receptor as a therapeutic target: patient selection and mechanisms of resistance to receptor-targeted drugs. J Clin Oncol. 2003; 21:23 Suppl. 289s–291s.
Article2. Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol. 2010; 7(9):493–507.
Article3. Peng D, Fan Z, Lu Y, DeBlasio T, Scher H, Mendelsohn J. Anti-epidermal growth factor receptor monoclonal antibody 225 up-regulates p27KIP1 and induces G1 arrest in prostatic cancer cell line DU145. Cancer Res. 1996; 56(16):3666–3669.4. Fagin JA. The Jeremiah Metzger lecture: intelligent design of cancer therapy: trials and tribulations. Trans Am Clin Climatol Assoc. 2007; 118:253–261.5. Perrotte P, Matsumoto T, Inoue K, Kuniyasu H, Eve BY, Hicklin DJ, Radinsky R, Dinney CPN. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999; 5(2):257–265.6. Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997; 151(6):1523–1530.7. Pepper C, Hoy T, Bentley DP. Bcl-2/Bax ratios in chronic lymphocytic leukaemia and their correlation with in vitro apoptosis and clinical resistance. Br J Cancer. 1997; 76(7):935–938.8. Wu X, Fan Z, Masui H, Rosen N, Mendelsohn J. Apoptosis induced by an anti-epidermal growth factor receptor monoclonal antibody in a human colorectal carcinoma cell line and its delay by insulin. J Clin Invest. 1995; 95(4):1897–1905.
Article9. Ciardiello F, Bianco R, Damiano V, De Lorenzo S, Pepe S, De Placido S, Fan Z, Mendelsohn J, Bianco AR, Tortora G. Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res. 1999; 5(4):909–916.10. Wild R, Fager K, Flefleh C, Kan D, Inigo I, Castaneda S, Luo FR, Camuso A, McGlinchey K, Rose WC. Cetuximab preclinical antitumor activity (monotherapy and combination based) is not predicted by relative total or activated epidermal growth factor receptor tumor expression levels. Mol Cancer Ther. 2006; 5(1):104–113.
Article11. Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004; 22(7):1201–1208.
Article12. Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004; 351(4):337–345.
Article13. Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 2008; 5(9):763–775.
Article14. Zhang J, Campbell RE, Ting AY, Tsien RY. Creating new fluorescent probes for cell biology. Nat Rev Mol Cell Biol. 2002; 3(12):906–918.
Article15. Waggoner A. Fluorescent labels for proteomics and genomics. Curr Opin Chem Biol. 2006; 10(1):62–66.
Article16. Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008; 49:2 Suppl. 113S–128S.
Article17. Barrett T, Koyama Y, Hama Y, Ravizzini G, Shin IS, Jang BS, Paik CH, Urano Y, Choyke PL, Kobayashi H. In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin Cancer Res. 2007; 13(22 Pt 1):6639–6648.18. Mendelsohn J. The epidermal growth factor receptor as a target for cancer therapy. Endocr Relat Cancer. 2001; 8(1):3–9.
Article19. Ma T, Liu H, Sun X, Gao L, Shi J, Zhao H, Jia B, Wang F, Liu Z. Serial in vivo imaging using a fluorescence probe allows identification of tumor early response to cetuximab immunotherapy. Mol Pharm. 2015; 12(1):10–17.20. Weissleder R, Tung CH, Mahmood U, Bogdanov A Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999; 17(4):375–378.21. Van Emburgh BO, Sartore-Bianchi A, Di Nicolantonio F, Siena S, Bardelli A. Acquired resistance to EGFR-targeted therapies in colorectal cancer. Mol Oncol. 2014; 8(6):1084–1094.22. Wunder A, Tung CH, Müller-Ladner U, Weissleder R, Mahmood U. In vivo imaging of protease activity in arthritis: a novel approach for monitoring treatment response. Arthritis Rheum. 2004; 50(8):2459–2465.23. Baselga J, Norton L, Masui H, Pandiella A, Coplan K, Miller WH Jr, Mendelsohn J. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst. 1993; 85(16):1327–1333.
Article24. Fan Z, Baselga J, Masui H, Mendelsohn J. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 1993; 53(19):4637–4642.25. Mukherjee P, Bhattacharya R, Wang P, Wang L, Basu S, Nagy JA, Atala A, Mukhopadhyay D, Soker S. Antiangiogenic properties of gold nanoparticles. Clin Cancer Res. 2005; 11(9):3530–3534.
Article26. Takata M, Chikumi H, Miyake N, Adachi K, Kanamori Y, Yamasaki A, Igishi T, Burioka N, Nanba E, Shimizu E. Lack of AKT activation in lung cancer cells with EGFR mutation is a novel marker of cetuximab sensitivity. Cancer Biol Ther. 2012; 13(6):369–378.
Article27. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995; 1(1):27–31.
Article28. Fonsatti E, Nicolay HJ, Altomonte M, Covre A, Maio M. Targeting cancer vasculature via endoglin/CD105: a novel antibody-based diagnostic and therapeutic strategy in solid tumours. Cardiovasc Res. 2010; 86(1):12–19.
Article29. Yang Y, Zhang Y, Hong H, Liu G, Leigh BR, Cai W. In vivo near-infrared fluorescence imaging of CD105 expression during tumor angiogenesis. Eur J Nucl Med Mol Imaging. 2011; 38(11):2066–2076.30. Bandyopadhyay D, Mandal M, Adam L, Mendelsohn J, Kumar R. Physical interaction between epidermal growth factor receptor and DNA-dependent protein kinase in mammalian cells. J Biol Chem. 1998; 273(3):1568–1573.
Article31. Tseng SH, Chou MY, Chu IM. Cetuximab-conjugated iron oxide nanoparticles for cancer imaging and therapy. Int J Nanomedicine. 2015; 10:3663–3685.32. Conde J, Bao C, Cui D, Baptista PV, Tian F. Antibody-drug gold nanoantennas with Raman spectroscopic fingerprints for in vivo tumour theranostics. J Control Release. 2014; 183:87–93.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Paronychia Occurring after Injection with Cetuximab (Erbitux(R), IMC-C225)
- Near-Infrared Contrast Agents for Bone-Targeted Imaging
- Assessment and Comparison of Three Dimensional Exoscopes for Near-Infrared Fluorescence-Guided Surgery Using Second-Window Indocyanine-Green
- Immunotherapy in Head and Neck Squamous Cell Cancer
- Identifying the Patterns of Adverse Drug Responses of Cetuximab