Clin Nutr Res.
2018 Jan;7(1):56-68. 10.7762/cnr.2018.7.1.56.
Dual Effects of High Protein Diet on Mouse Skin and Colonic Inflammation
- Affiliations
-
- 1Department of Food Science and Nutrition, Daegu Catholic University, Gyeongsan 38430, Korea. kimeunj@cu.ac.kr
- KMID: 2402343
- DOI: http://doi.org/10.7762/cnr.2018.7.1.56
Abstract
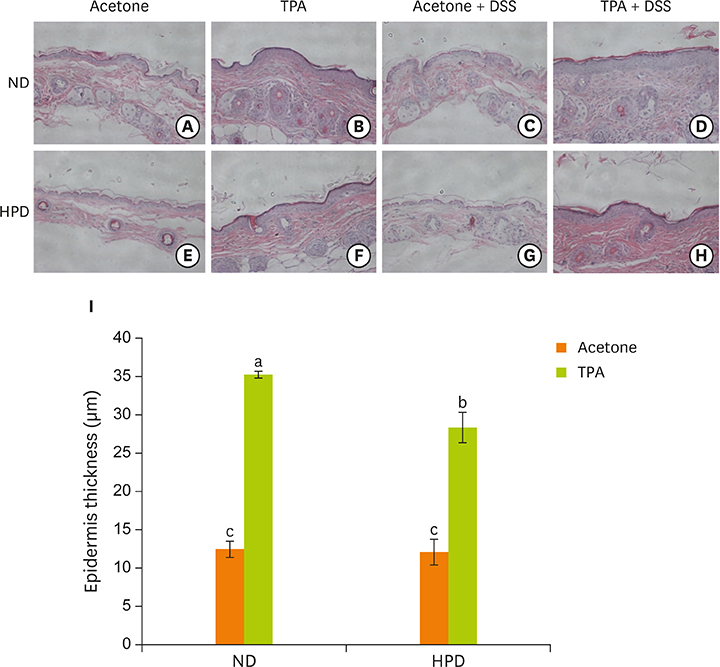
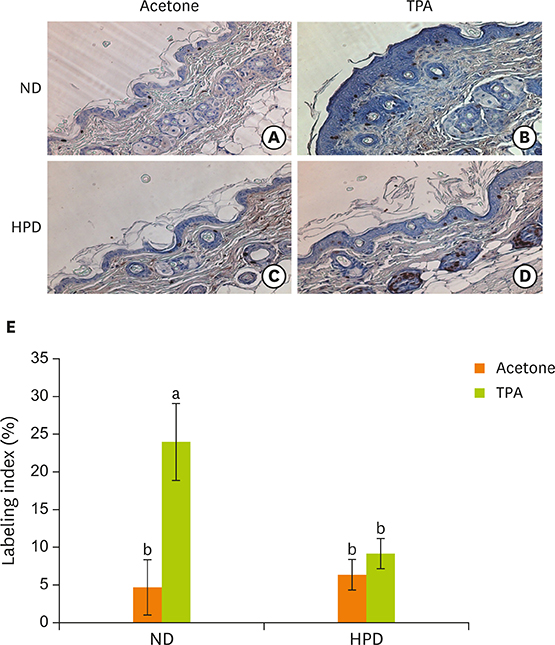
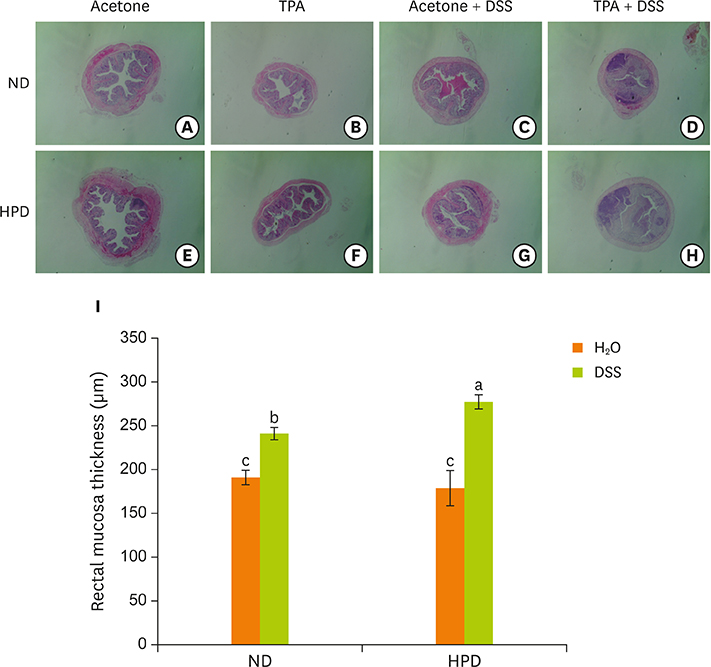
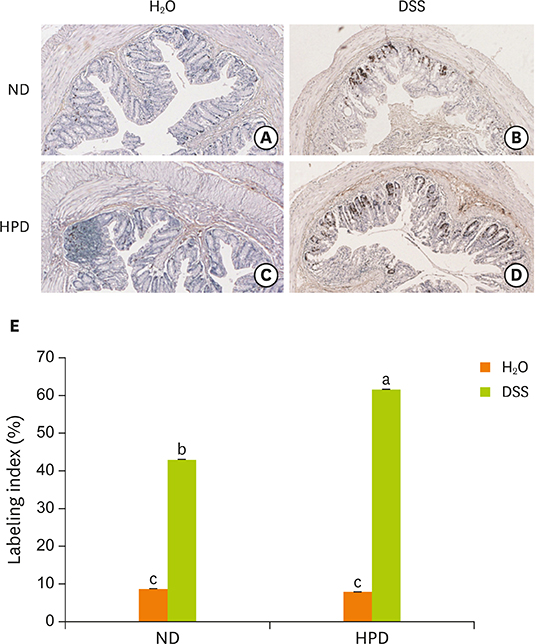
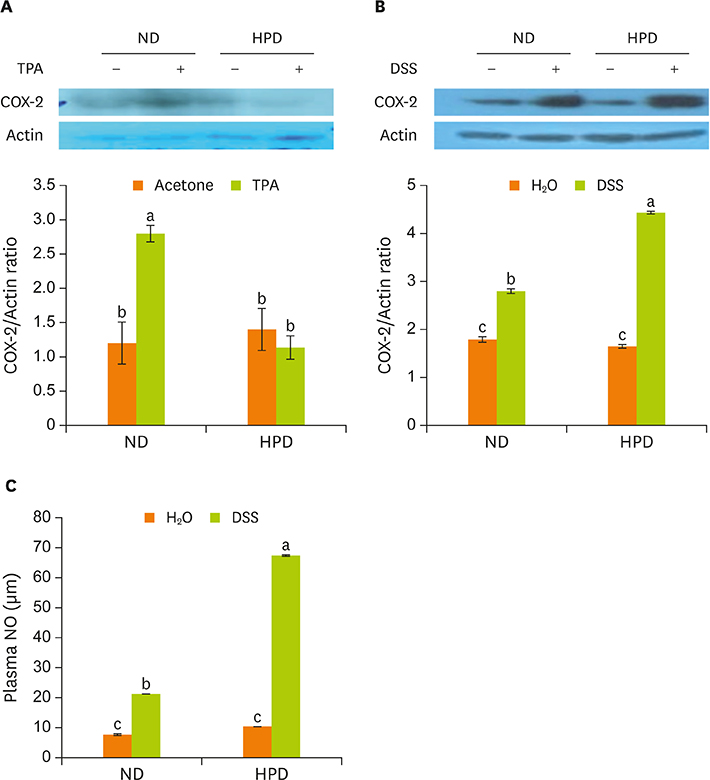
- Chronic inflammation is a major etiology of cancer. Accumulating epidemiological and experimental evidences suggest that intake of high protein diet (HPD) is associated with colitis-associated colon cancer, however, most of the studies were confined in colon. Systemic influence of HPD on inflammation indices in different tissues of an organism has never been studied. We therefore investigated the effect of HPD on mouse skin and colonic inflammation using the well characterized inflammation induction protocol in both tissues (12-O-tetradecanoylphorbol-13-acetate [TPA] for skin and dextran sodium sulfate [DSS] for colon). ICR mice were grouped to normal diet (ND, 20% casein) or HPD (50% casein) groups. In each diet group, mice were treated with either vehicle (acetone or Hâ‚‚O), TPA, TPA and DSS, or DSS. Experimental diet was fed for total 4 weeks. After 1 week of diet feeding, 6.5 nmol of TPA was topically applied twice a week for 2 weeks on the shaved mouse dorsal skin. Drinking water containing 2% DSS was administered for 7 days at the final week of experiment. The results showed that TPA-induced skin hyperplasia, epidermal cell proliferation, and cyclooxygenase-2 (COX-2) expression were reduced in HPD group compared to ND group. In contrast, HPD increased DSS-induced colon mucosal hyperplasia, colonocyte proliferation, COX-2 expression, and plasma nitric oxide compared to ND group. This suggests that HPD exerts differential effect on different tissue inflammation which implies efficacy of protein intervention to human also should be monitored more thoroughly.
Keyword
MeSH Terms
Figure
Reference
-
1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674.
Article2. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454:436–444.
Article3. Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005; 7:211–217.
Article4. Ogilvie GK. Interventional nutrition for the cancer patient. Clin Tech Small Anim Pract. 1998; 13:224–231.
Article5. Kurzer M, Meguid MM. Cancer and protein metabolism. Surg Clin North Am. 1986; 66:969–1001.
Article6. Kim E, Coelho D, Blachier F. Review of the association between meat consumption and risk of colorectal cancer. Nutr Res. 2013; 33:983–994.
Article7. Yao CK, Muir JG, Gibson PR. Review article: insights into colonic protein fermentation, its modulation and potential health implications. Aliment Pharmacol Ther. 2016; 43:181–196.
Article8. Lan A, Blais A, Coelho D, Capron J, Maarouf M, Benamouzig R, Lancha AH Jr, Walker F, Tomé D, Blachier F. Dual effects of a high-protein diet on DSS-treated mice during colitis resolution phase. Am J Physiol Gastrointest Liver Physiol. 2016; 311:G624–33.
Article9. Slaga TJ. Overview of tumor promotion in animals. Environ Health Perspect. 1983; 50:3–14.
Article10. Winberg LD, Budunova IV, Warren BS, Lyer RP, Slaga TJ. Mechanisms of skin tumor promotion and progression. In : Mukhtar H, editor. Skin cancer: mechanisms and human relevance. Boca Raton (FL): CRC Press;1995. p. 113–120.11. Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007; 28:1450–1459.
Article12. Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014; 18:279–288.
Article13. Tak KH, Kim E. Antitumorigenic effect of a high protein diet in mouse skin. J Food Sci Nutr. 2011; 16:283–290.
Article14. Byun SY, Kim DB, Kim E. Curcumin ameliorates the tumor-enhancing effects of a high-protein diet in an azoxymethane-induced mouse model of colon carcinogenesis. Nutr Res. 2015; 35:726–735.
Article15. Tak KH, Ahn E, Kim E. Increase in dietary protein content exacerbates colonic inflammation and tumorigenesis in azoxymethane-induced mouse colon carcinogenesis. Nutr Res Pract. 2017; 11:281–289.
Article16. Marelli G, Sica A, Vannucci L, Allavena P. Inflammation as target in cancer therapy. Curr Opin Pharmacol. 2017; 35:57–65.
Article17. Bozzetti F, Pagnoni AM, Del Vecchio M. Excessive caloric expenditure as a cause of malnutrition in patients with cancer. Surg Gynecol Obstet. 1980; 150:229–234.18. Dempsey DT, Mullen JL. Macronutrient requirements in the malnourished cancer patient. How much of what and why? Cancer. 1985; 55:290–294.
Article19. Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015; 7:17–29.
Article20. Niedel JE, Kuhn LJ, Vandenbark GR. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci USA. 1983; 80:36–40.
Article21. Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982; 257:7847–7851.
Article22. Marks F, Fürstenberger G. Cancer chemoprevention through interruption of multistage carcinogenesis. The lessons learnt by comparing mouse skin carcinogenesis and human large bowel cancer. Eur J Cancer. 2000; 36:314–329.
Article23. Tang F, Wang Y, Hemmings BA, Rüegg C, Xue G. PKB/Akt-dependent regulation of inflammation in cancer. Semin Cancer Biol. Forthcoming. 2017.
Article24. Lu J, Rho O, Wilker E, Beltran L, Digiovanni J. Activation of epidermal akt by diverse mouse skin tumor promoters. Mol Cancer Res. 2007; 5:1342–1352.
Article25. Segrelles C, Ruiz S, Perez P, Murga C, Santos M, Budunova IV, Martínez J, Larcher F, Slaga TJ, Gutkind JS, Jorcano JL, Paramio JM. Functional roles of Akt signaling in mouse skin tumorigenesis. Oncogene. 2002; 21:53–64.
Article26. Segrelles C, Lu J, Hammann B, Santos M, Moral M, Cascallana JL, Lara MF, Rho O, Carbajal S, Traag J, Beltrán L, Martínez-Cruz AB, García-Escudero R, Lorz C, Ruiz S, Bravo A, Paramio JM, DiGiovanni J. Deregulated activity of Akt in epithelial basal cells induces spontaneous tumors and heightened sensitivity to skin carcinogenesis. Cancer Res. 2007; 67:10879–10888.
Article27. Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993; 69:238–249.28. Cooper HS, Murthy S, Kido K, Yoshitake H, Flanigan A. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 2000; 21:757–768.
Article29. McIntosh GH, Le Leu RK. The influence of dietary proteins on colon cancer risk. Nutr Res. 2001; 21:1053–1066.
Article30. Bingham SA, Pignatelli B, Pollock JR, Ellul A, Malaveille C, Gross G, Runswick S, Cummings JH, O’Neill IK. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer? Carcinogenesis. 1996; 17:515–523.
Article31. Geypens B, Claus D, Evenepoel P, Hiele M, Maes B, Peeters M, Rutgeerts P, Ghoos Y. Influence of dietary protein supplements on the formation of bacterial metabolites in the colon. Gut. 1997; 41:70–76.
Article32. Silvester KR, Bingham SA, Pollock JR, Cummings JH, O’Neill IK. Effect of meat and resistant starch on fecal excretion of apparent N-nitroso compounds and ammonia from the human large bowel. Nutr Cancer. 1997; 29:13–23.
Article33. Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis. 2010; 16:684–695.
Article34. Liu X, Blouin JM, Santacruz A, Lan A, Andriamihaja M, Wilkanowicz S, Benetti PH, Tomé D, Sanz Y, Blachier F, Davila AM. High-protein diet modifies colonic microbiota and luminal environment but not colonocyte metabolism in the rat model: the increased luminal bulk connection. Am J Physiol Gastrointest Liver Physiol. 2014; 307:G459–70.
Article35. Toden S, Bird AR, Topping DL, Conlon MA. Resistant starch attenuates colonic DNA damage induced by higher dietary protein in rats. Nutr Cancer. 2005; 51:45–51.
Article36. Toden S, Bird AR, Topping DL, Conlon MA. Resistant starch prevents colonic DNA damage induced by high dietary cooked red meat or casein in rats. Cancer Biol Ther. 2006; 5:267–272.
Article37. Toden S, Bird AR, Topping DL, Conlon MA. High red meat diets induce greater numbers of colonic DNA double-strand breaks than white meat in rats: attenuation by high-amylose maize starch. Carcinogenesis. 2007; 28:2355–2362.
Article38. Toden S, Bird AR, Topping DL, Conlon MA. Differential effects of dietary whey, casein and soya on colonic DNA damage and large bowel SCFA in rats fed diets low and high in resistant starch. Br J Nutr. 2007; 97:535–543.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increase in dietary protein content exacerbates colonic inflammation and tumorigenesis in azoxymethane-induced mouse colon carcinogenesis
- Effects of luteolin on chemical induced colon carcinogenesis in high fat diet-fed obese mouse
- Differential effects of various dietary proteins on dextran sulfate sodiuminduced colitis in mice
- Inhibition of Phorbol Ester-induced Mouse Skin Tumor Promotion and COX-2 Expression by Celecoxib: C/EBP as a Potential Molecular Target
- Influences of Various Factors on Changes in Concentration of the Prostatic Citrate