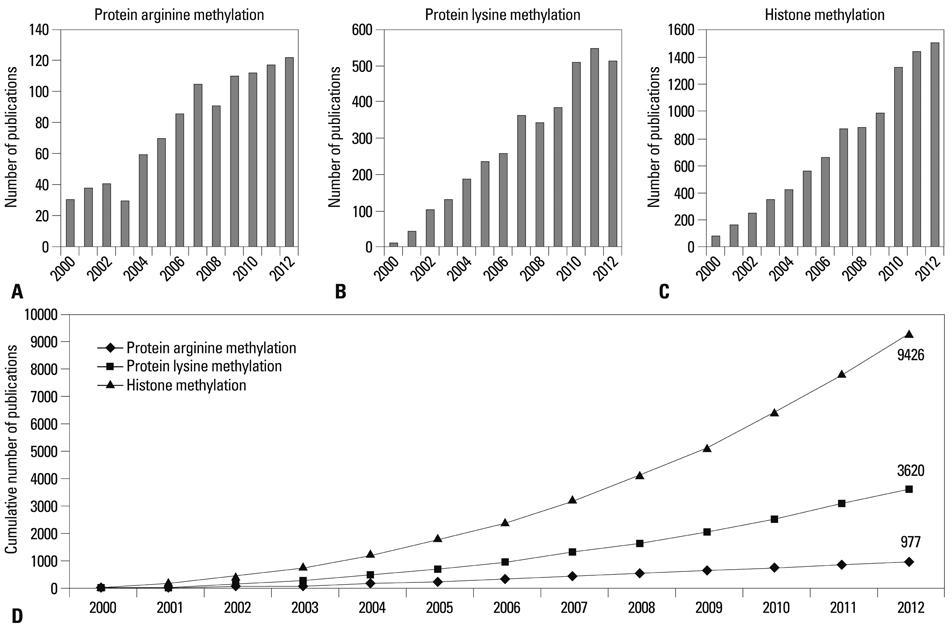
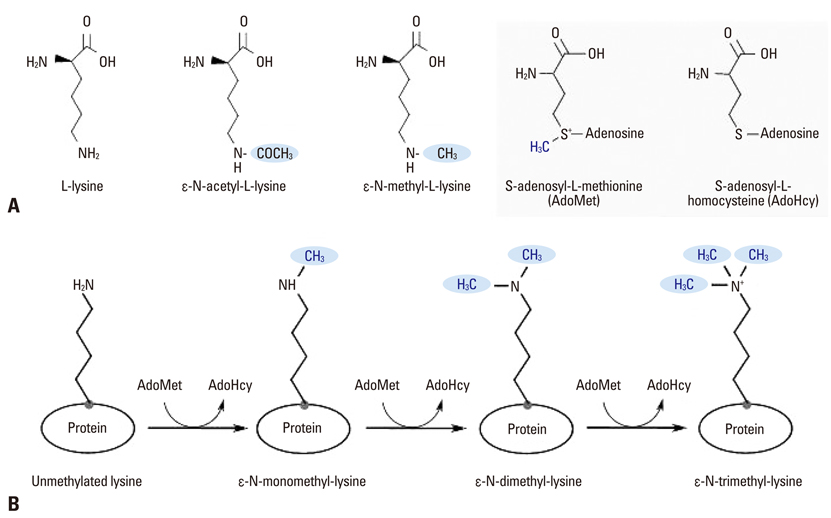
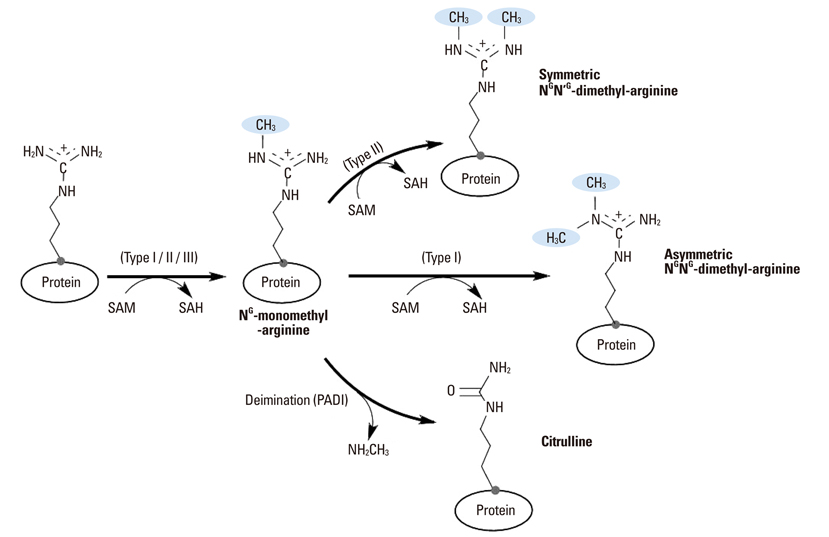
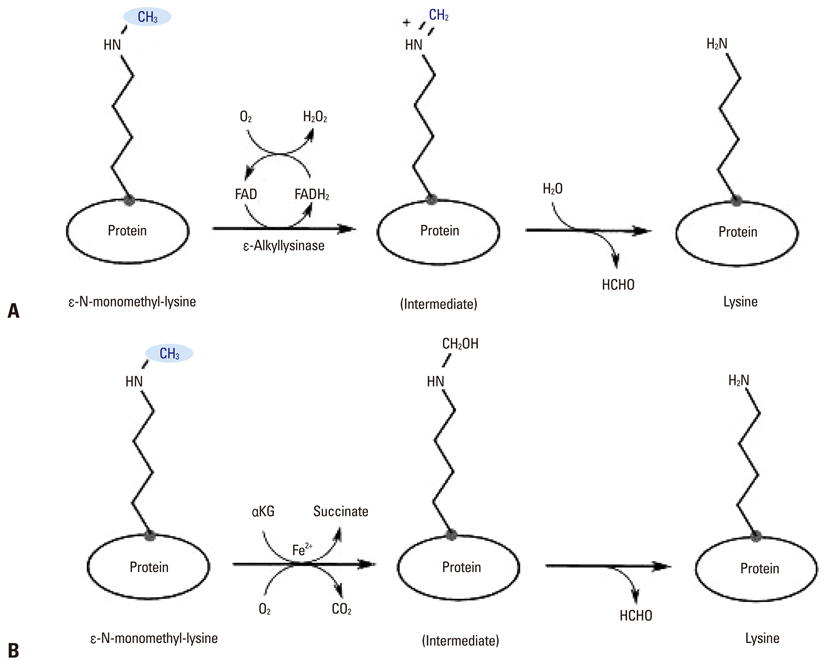
Yonsei Med J.
2014 Mar;55(2):292-303.
Protein Methylation and Interaction with the Antiproliferative Gene, BTG2(/TIS21/Pc3)
- Affiliations
-
- 1Temple University School of Medicine, Philadelphia, PA, USA. sdkim7818@temple.edu
- 2Department of Biochemistry and Molecular Biology, Ajou University School of Medicine, Suwon, Korea. iklim@ajou.ac.kr
Abstract
- The last one and half a decade witnessed an outstanding re-emergence of attention and remarkable progress in the field of protein methylation. In the present article, we describe the early discoveries in research and review the role protein methylation played in the biological function of the antiproliferative gene, BTG2(/TIS21/PC3).
Keyword
Figure
Reference
-
1. Kim S, Paik WK. Studies on the origin of epsilon-N-methyl-L-lysine in protein. J Biol Chem. 1965; 240:4629–4634.2. Schoenheimer R, Ratner S, Rittenberg D. Studies on protein metabolism: VII. The metabolism of tyrosine. J Biol Chem. 1939; 127:333–344.3. Neuberger A, Sanger F. The availability of in-acetyl-d-lysine and in-methyl-dl-lysine for growth. Biochem J. 1944; 38:125–129.4. Ambler RP, Rees MW. Epsilon-N-methyl-lysine in bacterial flagellar protein. Nature. 1959; 184:56–57.5. Murray K. The occurence of epsilon-N-methyl lysine in histones. Biochemistry. 1964; 3:10–15.6. Huang RC, Bonner J. Histone, a suppressor of chromosomal RNA synthesis. Proc Natl Acad Sci U S A. 1962; 48:1216–1222.
Article7. Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci U S A. 1964; 51:786–794.
Article8. Paik WK, Bloch-Frankenthal L, Birnbaum SM, Winitz M, Greenstein JP. Eepsilon-lysine acylase. Arch Biochem Biophys. 1957; 69:56–66.9. Kim S, Benoiton L, Paik WK. On the metabolism of epsilon-N-methyl-L-lysine by rat-kidney homogenate. Biochim Biophys Acta. 1963; 71:745–747.10. Kim S, Benoition L, Paik WK. Epsilon-alkyllysinase. Purification and properties of the enzyme. J Biol Chem. 1964; 239:3790–3796.11. Paik WK, Kim S. Epsilon-alkyllysinase. New assay method, purification, and biological significance. Arch Biochem Biophys. 1974; 165:369–378.
Article12. Mosammaparast N, Shi Y. Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu Rev Biochem. 2010; 79:155–179.
Article13. Paik WK, Kim S. ε-N-dimethyllysine in histones. Biochem Biophys Res Commun. 1967; 27:216–220.14. Hempel K, Lange HW, Birkofer L. [Epsilon-N-trimethyllysine, a new amino acid in histones]. Naturwissenschaften. 1968; 55:37.15. Takemoto T, Daigo K, Takagi N. [Studies on the hypotensive constituents of marine algae. I. a new basic amino acid "laminine" and the other basic constituents isolated from laminaria angustata]. Yakugaku Zasshi. 1964; 84:1176–1179.
Article16. Paik WK, Kim S. Protein methylase I. Purification and properties of the enzyme. J Biol Chem. 1968; 243:2108–2114.17. Kakimoto Y, Akazawa S. Isolation and identification of N-G,N-G- and N-G,N'-G-dimethyl-arginine, N-epsilon-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine. J Biol Chem. 1970; 245:5751–5758.
Article18. Baldwin GS, Carnegie PR. Specific enzymic methylation of an arginine in the experimental allergic encephalomyelitis protein from human myelin. Science. 1971; 171:579–581.
Article19. Brostoff S, Eylar EH. Localization of methylated arginine in the A1 protein from myelin. Proc Natl Acad Sci U S A. 1971; 68:765–769.
Article20. Rajpurohit R, Lee SO, Park JO, Paik WK, Kim S. Enzymatic methylation of recombinant heterogeneous nuclear RNP protein A1. Dual substrate specificity for S-adenosylmethionine:histone-arginine N-methyltransferase. J Biol Chem. 1994; 269:1075–1082.
Article21. Ghosh SK, Paik WK, Kim S. Purification and molecular identification of two protein methylases I from calf brain. Myelin basic protein- and histone-specific enzyme. J Biol Chem. 1988; 263:19024–19033.
Article22. Wang YC, Li C. Evolutionarily conserved protein arginine methyltransferases in non-mammalian animal systems. FEBS J. 2012; 279:932–945.
Article23. Kim S, Paik WK. Purification and properties of protein methylase II. J Biol Chem. 1970; 245:1806–1813.
Article24. Liss M, Edelstein LM. Evidence for the enzymatic methylation of crystalline ovalbumin preparations. Biochem Biophys Res Commun. 1967; 26:497–504.
Article25. Axelrod J, Daly J. Pituitary gland: enzymic formation of methanol from S-adenosylmethionine. Science. 1965; 150:892–893.
Article26. Springer MS, Goy MF, Adler J. Protein methylation in behavioural control mechanisms and in signal transduction. Nature. 1979; 280:279–284.
Article27. Clarke S. Aging as war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair. Ageing Res Rev. 2003; 2:263–285.
Article28. Paik WK, Kim S. Solubilization and partial purification of protein methylase 3 from calf thymus nuclei. J Biol Chem. 1970; 245:6010–6015.
Article29. Paik WK, Kim S. Protein methylation. Science. 1971; 174:114–119.
Article30. Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005; 6:838–849.
Article31. Fackelmayer FO. Protein arginine methyltransferases: guardians of the Arg? Trends Biochem Sci. 2005; 30:666–671.
Article32. Pahlich S, Zakaryan RP, Gehring H. Protein arginine methylation: Cellular functions and methods of analysis. Biochim Biophys Acta. 2006; 1764:1890–1903.
Article33. Lee DY, Teyssier C, Strahl BD, Stallcup MR. Role of protein methylation in regulation of transcription. Endocr Rev. 2005; 26:147–170.
Article34. Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Mol Cell. 2005; 18:263–272.35. Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004; 119:941–953.
Article36. Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004; 306:279–283.
Article37. Cuthbert GL, Daujat S, Snowden AW, Erdjument-Bromage H, Hagiwara T, Yamada M, et al. Histone deimination antagonizes arginine methylation. Cell. 2004; 118:545–553.
Article38. Teyssier C, Le Romancer M, Sentis S, Jalaguier S, Corbo L, Cavaillès V. Protein arginine methylation in estrogen signaling and estrogen-related cancers. Trends Endocrinol Metab. 2010; 21:181–189.
Article39. Yang Y, Bedford MT. Protein arginine methyltransferases and cancer. Nat Rev Cancer. 2013; 13:37–50.
Article40. Blackwell E, Ceman S. Arginine methylation of RNA-binding proteins regulates cell function and differentiation. Mol Reprod Dev. 2012; 79:163–175.
Article41. Ahmad A, Cao X. Plant PRMTs broaden the scope of arginine methylation. J Genet Genomics. 2012; 39:195–208.
Article42. Fisk JC, Read LK. Protein arginine methylation in parasitic protozoa. Eukaryot Cell. 2011; 10:1013–1022.
Article43. Parry RV, Ward SG. Protein arginine methylation: a new handle on T lymphocytes? Trends Immunol. 2010; 31:164–169.
Article44. Kim S, Lim IK, Park GH, Paik WK. Biological methylation of myelin basic protein: enzymology and biological significance. Int J Biochem Cell Biol. 1997; 29:743–751.
Article45. An W, Kim J, Roeder RG. Ordered cooperative functions of PRMT1, p300, and CARM1 in transcriptional activation by p53. Cell. 2004; 117:735–748.
Article46. Leiper J, Nandi M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discov. 2011; 10:277–291.
Article47. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–142.48. Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006; 439:811–816.
Article49. Yamane K, Toumazou C, Tsukada Y, Erdjument-Bromage H, Tempst P, Wong J, et al. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006; 125:483–495.
Article50. Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, et al. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006; 125:467–481.
Article51. Paik WK, Kim S. Protein methylation. Boca Raton, FL: CRC Press;1990.52. Raunser S, Magnani R, Huang Z, Houtz RL, Trievel RC, Penczek PA, et al. Rubisco in complex with Rubisco large subunit methyltransferase. Proc Natl Acad Sci U S A. 2009; 106:3160–3165.
Article53. Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012; 13:343–357.
Article54. Yap KL, Zhou MM. Structure and mechanisms of lysine methylation recognition by the chromodomain in gene transcription. Biochemistry. 2011; 50:1966–1980.
Article55. Beck DB, Oda H, Shen SS, Reinberg D. PR-Set7 and H4K20me1: at the crossroads of genome integrity, cell cycle, chromosome condensation, and transcription. Genes Dev. 2012; 26:325–337.
Article56. Han S, Brunet A. Histone methylation makes its mark on longevity. Trends Cell Biol. 2012; 22:42–49.
Article57. Sen GL. Remembering one's identity: the epigenetic basis of stem cell fate decisions. FASEB J. 2011; 25:2123–2128.
Article58. Miller D, Brinkworth M, Iles D. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction. 2010; 139:287–301.
Article59. Wagner EJ, Carpenter PB. Understanding the language of Lys36 methylation at histone H3. Nat Rev Mol Cell Biol. 2012; 13:115–126.
Article60. Holec S, Berger F. Polycomb group complexes mediate developmental transitions in plants. Plant Physiol. 2012; 158:35–43.
Article61. Thorstensen T, Grini PE, Aalen RB. SET domain proteins in plant development. Biochim Biophys Acta. 2011; 1809:407–420.
Article62. Keating ST, El-Osta A. Chromatin modifications associated with diabetes. J Cardiovasc Transl Res. 2012; 5:399–412.
Article63. Imai K, Ochiai K. Role of histone modification on transcriptional regulation and HIV-1 gene expression: possible mechanisms of periodontal diseases in AIDS progression. J Oral Sci. 2011; 53:1–13.
Article64. Peter CJ, Akbarian S. Balancing histone methylation activities in psychiatric disorders. Trends Mol Med. 2011; 17:372–379.
Article65. Paik WK, Nochumson S, Kim S. Carnitine biosynthesis via protein methylation. Trens Biochem Sci. 1977; 2:159–161.
Article66. Matsuda S, Rouault J, Magaud J, Berthet C. In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Lett. 2001; 497:67–72.
Article67. Buanne P, Corrente G, Micheli L, Palena A, Lavia P, Spadafora C, et al. Cloning of PC3B, a novel member of the PC3/BTG/TOB family of growth inhibitory genes, highly expressed in the olfactory epithelium. Genomics. 2000; 68:253–263.
Article68. Guéhenneux F, Duret L, Callanan MB, Bouhas R, Hayette S, Berthet C, et al. Cloning of the mouse BTG3 gene and definition of a new gene family (the BTG family) involved in the negative control of the cell cycle. Leukemia. 1997; 11:370–375.
Article69. Fletcher BS, Lim RW, Varnum BC, Kujubu DA, Koski RA, Herschman HR. Structure and expression of TIS21, a primary response gene induced by growth factors and tumor promoters. J Biol Chem. 1991; 266:14511–14518.
Article70. Bradbury A, Possenti R, Shooter EM, Tirone F. Molecular cloning of PC3, a putatively secreted protein whose mRNA is induced by nerve growth factor and depolarization. Proc Natl Acad Sci U S A. 1991; 88:3353–3357.
Article71. Konrad MA, Zúñiga-Pflücker JC. The BTG/TOB family protein TIS21 regulates stage-specific proliferation of developing thymocytes. Eur J Immunol. 2005; 35:3030–3042.
Article72. Kim BC, Ryu MS, Oh SP, Lim IK. TIS21/(BTG2) negatively regulates estradiol-stimulated expansion of hematopoietic stem cells by derepressing Akt phosphorylation and inhibiting mTOR signal transduction. Stem Cells. 2008; 26:2339–2348.
Article73. Ryu MS, Lee MS, Hong JW, Hahn TR, Moon E, Lim IK. TIS21/BTG2/PC3 is expressed through PKC-delta pathway and inhibits binding of cyclin B1-Cdc2 and its activity, independent of p53 expression. Exp Cell Res. 2004; 299:159–170.
Article74. Guardavaccaro D, Corrente G, Covone F, Micheli L, D'Agnano I, Starace G, et al. Arrest of G(1)-S progression by the p53-inducible gene PC3 is Rb dependent and relies on the inhibition of cyclin D1 transcription. Mol Cell Biol. 2000; 20:1797–1815.
Article75. Lim IK, Lee MS, Ryu MS, Park TJ, Fujiki H, Eguchi H, et al. Induction of growth inhibition of 293 cells by downregulation of the cyclin E and cyclin-dependent kinase 4 proteins due to overexpression of TIS21. Mol Carcinog. 1998; 23:25–35.
Article76. Hong JW, Ryu MS, Lim IK. Phosphorylation of serine 147 of tis21/BTG2/pc3 by p-Erk1/2 induces Pin-1 binding in cytoplasm and cell death. J Biol Chem. 2005; 280:21256–21263.
Article77. Lim YB, Park TJ, Lim IK. B cell translocation gene 2 enhances susceptibility of HeLa cells to doxorubicin-induced oxidative damage. J Biol Chem. 2008; 283:33110–33118.
Article78. Corrente G, Guardavaccaro D, Tirone F. PC3 potentiates NGF-induced differentiation and protects neurons from apoptosis. Neuroreport. 2002; 13:417–422.
Article79. Farioli-Vecchioli S, Saraulli D, Costanzi M, Leonardi L, Cinà I, Micheli L, et al. Impaired terminal differentiation of hippocampal granule neurons and defective contextual memory in PC3/Tis21 knockout mice. PLoS One. 2009; 4:e8339.
Article80. Passeri D, Marcucci A, Rizzo G, Billi M, Panigada M, Leonardi L, et al. Btg2 enhances retinoic acid-induced differentiation by modulating histone H4 methylation and acetylation. Mol Cell Biol. 2006; 26:5023–5032.
Article81. Imran M, Park TJ, Lim IK. TIS21/BTG2/PC3 enhances downregulation of c-Myc during differentiation of HL-60 cells by activating Erk1/2 and inhibiting Akt in response to all-trans-retinoic acid. Eur J Cancer. 2012; 48:2474–2485.
Article82. Tirone F. The gene PC3(TIS21/BTG2), prototype member of the PC3/BTG/TOB family: regulator in control of cell growth, differentiation, and DNA repair? J Cell Physiol. 2001; 187:155–165.
Article83. Choi KS, Kim JY, Lim SK, Choi YW, Kim YH, Kang SY, et al. TIS21(/BTG2/PC3) accelerates the repair of DNA double strand breaks by enhancing Mre11 methylation and blocking damage signal transfer to the Chk2(T68)-p53(S20) pathway. DNA Repair (Amst). 2012; 11:965–975.
Article84. Lim SK, Choi YW, Lim IK, Park TJ. BTG2 suppresses cancer cell migration through inhibition of Src-FAK signaling by downregulation of reactive oxygen species generation in mitochondria. Clin Exp Metastasis. 2012; 29:901–913.
Article85. Karve TM, Rosen EM. B-cell translocation gene 2 (BTG2) stimulates cellular antioxidant defenses through the antioxidant transcription factor NFE2L2 in human mammary epithelial cells. J Biol Chem. 2012; 287:31503–31514.
Article86. Herschman HR. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991; 60:281–319.
Article87. Imran M, Lim IK. Regulation of Btg2(/TIS21/PC3) expression via reactive oxygen species-protein kinase C-NFκB pathway under stress conditions. Cell Signal. 2013; 25:2400–2412.
Article88. Lin WJ, Gary JD, Yang MC, Clarke S, Herschman HR. The mammalian immediate-early TIS21 protein and the leukemia-associated BTG1 protein interact with a protein-arginine N-methyltransferase. J Biol Chem. 1996; 271:15034–15044.
Article89. Berthet C, Guéhenneux F, Revol V, Samarut C, Lukaszewicz A, Dehay C, et al. Interaction of PRMT1 with BTG/TOB proteins in cell signalling: molecular analysis and functional aspects. Genes Cells. 2002; 7:29–39.
Article90. Lim IK, Park TJ, Kim S, Lee HW, Paik WK. Enzymatic methylation of recombinant TIS21 protein-arginine residues. Biochem Mol Biol Int. 1998; 45:871–878.
Article91. Bakker WJ, Blázquez-Domingo M, Kolbus A, Besooyen J, Steinlein P, Beug H, et al. FoxO3a regulates erythroid differentiation and induces BTG1, an activator of protein arginine methyl transferase 1. J Cell Biol. 2004; 164:175–184.
Article92. Miyata S, Mori Y, Tohyama M. PRMT1 and Btg2 regulates neurite outgrowth of Neuro2a cells. Neurosci Lett. 2008; 445:162–165.
Article93. Hata K, Nishijima K, Mizuguchi J. Role for Btg1 and Btg2 in growth arrest of WEHI-231 cells through arginine methylation following membrane immunoglobulin engagement. Exp Cell Res. 2007; 313:2356–2366.
Article94. Zhang J, Tsaprailis G, Bowden GT. Nucleolin stabilizes Bcl-X L messenger RNA in response to UVA irradiation. Cancer Res. 2008; 68:1046–1054.
Article95. Mauxion F, Faux C, Séraphin B. The BTG2 protein is a general activator of mRNA deadenylation. EMBO J. 2008; 27:1039–1048.
Article96. Rouault JP, Prévôt D, Berthet C, Birot AM, Billaud M, Magaud JP, et al. Interaction of BTG1 and p53-regulated BTG2 gene products with mCaf1, the murine homolog of a component of the yeast CCR4 transcriptional regulatory complex. J Biol Chem. 1998; 273:22563–22569.
Article97. Robin-Lespinasse Y, Sentis S, Kolytcheff C, Rostan MC, Corbo L, Le Romancer M. hCAF1, a new regulator of PRMT1-dependent arginine methylation. J Cell Sci. 2007; 120(Pt 4):638–647.
Article98. Collart MA, Panasenko OO. The Ccr4--not complex. Gene. 2012; 492:42–53.
Article99. Boisvert FM, Déry U, Masson JY, Richard S. Arginine methylation of MRE11 by PRMT1 is required for DNA damage checkpoint control. Genes Dev. 2005; 19:671–676.
Article100. Stracker TH, Petrini JH. The MRE11 complex: starting from the ends. Nat Rev Mol Cell Biol. 2011; 12:90–103.
Article101. Déry U, Coulombe Y, Rodrigue A, Stasiak A, Richard S, Masson JY. A glycine-arginine domain in control of the human MRE11 DNA repair protein. Mol Cell Biol. 2008; 28:3058–3069.
Article102. Yu Z, Vogel G, Coulombe Y, Dubeau D, Spehalski E, Hébert J, et al. The MRE11 GAR motif regulates DNA double-strand break processing and ATR activation. Cell Res. 2012; 22:305–320.
Article103. Chrzanowska KH, Gregorek H, Dembowska-Bagińska B, Kalina MA, Digweed M. Nijmegen breakage syndrome (NBS). Orphanet J Rare Dis. 2012; 7:13.
Article104. Lehninger AL. Biochemistry: The molecular basis of cell structure and function. New York, NY: Worth Publisher;1970.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of TNFα-interacting protein α (Tipα)-associated gastric carcinogenesis by BTG2(/TIS21) via downregulating cytoplasmic nucleolin expression
- B-cell translocation gene 2 positively regulates GLP-1-stimulated insulin secretion via induction of PDX-1 in pancreatic beta-cells
- DNA Methylation in Development
- Methylation of P16 and hMLH1 in Gastric Carcinoma
- In silico Identification of SFRP1 as a Hypermethylated Gene in Colorectal Cancers