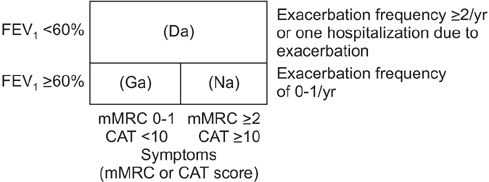
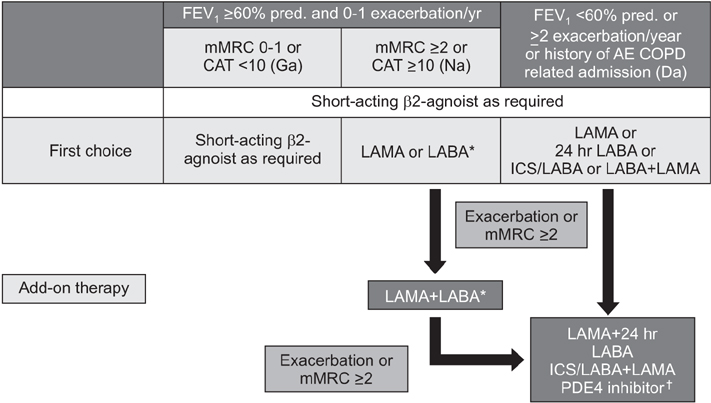
Summary of the Chronic Obstructive Pulmonary Disease Clinical Practice Guideline Revised in 2014 by the Korean Academy of Tuberculosis and Respiratory Disease
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Hallym University Kangdong Sacred Heart Hospital, Seoul, Korea.
- 3Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea.
- 5Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ymoh55@amc.seoul.kr
- KMID: 2385037
- DOI: http://doi.org/10.4046/trd.2017.80.3.230
Abstract
- Chronic obstructive pulmonary disease (COPD) results in high morbidity and mortality among patients both domestically and globally. The Korean clinical practice guideline for COPD was revised in 2014. It was drafted by the members of the Korean Academy of Tuberculosis and Respiratory Diseases, as well as participating members of the Health Insurance Review and Assessment Service, Korean Physicians' Association, and Korea Respiration Trouble Association. This revised guideline covers a wide range of topics, including the epidemiology, diagnosis, assessment, monitoring, management, exacerbation, and comorbidities of COPD in Korea. We drafted a guideline on COPD management by performing systematic reviews on the topic of management with the help of a meta-analysis expert. We expect this guideline will be helpful medical doctors treating patients with respiratory conditions, other health care professionals, and government personnel in South Korea.
MeSH Terms
Figure
Cited by 5 articles
-
Increased Risk of Exacerbation in Asthma Predominant Asthma–Chronic Obstructive Pulmonary Disease Overlap Syndrome
Jisoo Park, Eun-Kyung Kim, Mi-Ae Kim, Tae-Hyung Kim, Jung Hyun Chang, Yon Ju Ryu, Sei Won Lee, Yeon-Mok Oh, Suk Joong Yong, Won-Il Choi, Kwang Ha Yoo, Ji-Hyun Lee
Tuberc Respir Dis. 2018;81(4):289-298. doi: 10.4046/trd.2017.0064.What Can We Apply to Manage Acute Exacerbation of Chronic Obstructive Pulmonary Disease with Acute Respiratory Failure?
Deog Kyeom Kim, Jungsil Lee, Ju-Hee Park, Kwang Ha Yoo
Tuberc Respir Dis. 2018;81(2):99-105. doi: 10.4046/trd.2017.0094.The diagnosis of chronic obstructive pulmonary disease according to current guidelines
Hyun Jung Kim, Yeon-Mok Oh
J Korean Med Assoc. 2018;61(9):539-544. doi: 10.5124/jkma.2018.61.9.539.Optimal Bronchodilation for COPD Patients: Are All Long-Acting β2-Agonist/Long-Acting Muscarinic Antagonists the Same?
Marc Miravitlles, Seungjae Baek, Vatsal Vithlani, Rahul Lad
Tuberc Respir Dis. 2018;81(3):198-215. doi: 10.4046/trd.2018.0040.Direct and Indirect Costs of Chronic Obstructive Pulmonary Disease in Korea
Changhwan Kim, Younhee Kim, Dong-Wook Yang, Chin Kook Rhee, Sung Kyoung Kim, Yong-Il Hwang, Yong Bum Park, Young Mok Lee, Seonglim Jin, Jinkyeong Park, Cho-Rom Hahm, Chang-Han Park, So Yeon Park, Cheol Kweon Jung, Yu-Il Kim, Sang Haak Lee, Hyoung Kyu Yoon, Jin Hwa Lee, Seong Yong Lim, Kwang Ha Yoo
Tuberc Respir Dis. 2019;82(1):27-34. doi: 10.4046/trd.2018.0035.
Reference
-
1. Kim C, Yoo KH, Rhee CK, Yoon HK, Kim YS, Lee SW, et al. Health care use and economic burden of patients with diagnosed chronic obstructive pulmonary disease in Korea. Int J Tuberc Lung Dis. 2014; 18:737–743.2. Statistics Korea. The result of causes of death statistics in 2010. Daejeon: Statistics Korea;2011.3. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006; 3:e442.4. Korean Academy of Tuberculosis and Respiratory Diseases. COPD clinical practice guidelines revised in 2014 [Internet]. Seoul: Korean Academy of Tuberculosis and Respiratory Diseases;2014. cited 2017 May 1. Available from: http://www.lungkorea.org/bbs/index.html?code=guide&page=2.5. Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006; 27:397–412.6. Bousquet J, Kiley J, Bateman ED, Viegi G, Cruz AA, Khaltaev N, et al. Prioritised research agenda for prevention and control of chronic respiratory diseases. Eur Respir J. 2010; 36:995–1001.7. Yoo KH, Kim YS, Sheen SS, Park JH, Hwang YI, Kim SH, et al. Prevalence of chronic obstructive pulmonary disease in Korea: the fourth Korean National Health and Nutrition Examination Survey, 2008. Respirology. 2011; 16:659–665.8. Statistics Korea. 2010 Morbidity statistics on causes of death among Koreans. Daejeon: Statistics Korea;2011.9. Oh IH, Yoon SJ, Kim EJ. The burden of disease in Korea. J Korean Med Assoc. 2011; 54:646–652.10. Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011; 139:752–763.11. Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, et al. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010; 182:693–718.12. Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003; 22:672–688.13. Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187:347–365.14. Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000; 320:1297–1303.15. Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994; 272:1497–1505.16. Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999; 353:1819–1823.17. Sestini P, Cappiello V, Aliani M, Martucci P, Sena A, Vaghi A, et al. Prescription bias and factors associated with improper use of inhalers. J Aerosol Med. 2006; 19:127–136.18. Donohue JF, Fogarty C, Lotvall J, Mahler DA, Worth H, Yorgancioglu A, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010; 182:155–162.19. Kornmann O, Dahl R, Centanni S, Dogra A, Owen R, Lassen C, et al. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J. 2011; 37:273–279.20. Barr RG, Bourbeau J, Camargo CA, Ram FS. Inhaled tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005; (2):CD002876.21. Kesten S, Casaburi R, Kukafka D, Cooper CB. Improvement in self-reported exercise participation with the combination of tiotropium and rehabilitative exercise training in COPD patients. Int J Chron Obstruct Pulmon Dis. 2008; 3:127–136.22. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359:1543–1554.23. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; (9):CD009157.24. Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Molken MP, Beeh KM, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011; 364:1093–1103.25. Jones PW, Singh D, Bateman ED, Agusti A, Lamarca R, de Miquel G, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012; 40:830–836.26. Kerwin E, Hebert J, Gallagher N, Martin C, Overend T, Alagappan VK, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur Respir J. 2012; 40:1106–1114.27. Beier J, Kirsten AM, Mroz R, Segarra R, Chuecos F, Caracta C, et al. Efficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease: results from a 6-week, randomized, controlled Phase IIIb study. COPD. 2013; 10:511–522.28. D'Urzo A, Kerwin E, Rennard S, He T, Gil EG, Caracta C. One-year extension study of ACCORD COPD I: safety and efficacy of two doses of twice-daily aclidinium bromide in patients with COPD. COPD. 2013; 10:500–510.29. Gelb AF, Tashkin DP, Make BJ, Zhong X, Garcia Gil E, Caracta C, et al. Long-term safety and efficacy of twice-daily aclidinium bromide in patients with COPD. Respir Med. 2013; 107:1957–1965.30. Beeh KM, Singh D, Di Scala L, Drollmann A. Once-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trial. Int J Chron Obstruct Pulmon Dis. 2012; 7:503–513.31. Chapman KR, Beeh KM, Beier J, Bateman ED, D'Urzo A, Nutbrown R, et al. A blinded evaluation of the efficacy and safety of glycopyrronium, a once-daily long-acting muscarinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 study. BMC Pulm Med. 2014; 14:4.32. D'Urzo A, Kerwin E, Overend T, D'Andrea P, Chen H, Goyal P. Once daily glycopyrronium for the treatment of COPD: pooled analysis of the GLOW1 and GLOW2 studies. Curr Med Res Opin. 2014; 30:493–508.33. ZuWallack RL, Mahler DA, Reilly D, Church N, Emmett A, Rickard K, et al. Salmeterol plus theophylline combination therapy in the treatment of COPD. Chest. 2001; 119:1661–1670.34. Rodrigo GJ, Plaza V. Efficacy and safety of a fixed-dose combination of indacaterol and Glycopyrronium for the treatment of COPD: a systematic review. Chest. 2014; 146:309–317.35. Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandstrom T, Taylor AF, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013; 1:199–209.36. Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003; 21:74–81.37. Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003; 361:449–456.38. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356:775–789.39. Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999; 340:1948–1953.40. Karner C, Cates CJ. Combination inhaled steroid and long-acting beta(2)-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011; (3):CD008532.41. Rabe KF, Bateman ED, O'Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast: an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005; 366:563–571.42. Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009; 374:685–694.43. Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007; 176:154–161.44. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009; 374:695–703.45. Rice KL, Kronenberg RS, Hedemark LL, Niewoehner DE. Effects of chronic administration of codeine and promethazine on breathlessness and exercise tolerance in patients with chronic airflow obstruction. Br J Dis Chest. 1987; 81:287–292.46. Chong J, Poole P, Leung B, Black PN. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011; (5):CD002309.47. Bateman ED, Rabe KF, Calverley PM, Goehring UM, Brose M, Bredenbroker D, et al. Roflumilast with long-acting beta2-agonists for COPD: influence of exacerbation history. Eur Respir J. 2011; 38:553–560.48. Lee SD, Hui DS, Mahayiddin AA, Roa CC Jr, Kwa KH, Goehring UM, et al. Roflumilast in Asian patients with COPD: a randomized placebo-controlled trial. Respirology. 2011; 16:1249–1257.49. Ni W, Shao X, Cai X, Wei C, Cui J, Wang R, et al. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. PLoS One. 2015; 10:e0121257.50. Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008; 178:1139–1147.51. Sethi S, Jones PW, Theron MS, Miravitlles M, Rubinstein E, Wedzicha JA, et al. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010; 11:10.52. Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA Jr, Criner GJ, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011; 365:689–698.53. Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006; 173:1390–1413.54. Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B. Long-term Oxygen Treatment Trial Research Group. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest. 2010; 138:179–187.55. Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010; 363:1233–1244.56. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD [Internet]. Global Initiative for Chronic Obstructive Lung Disease;2015. cited 2015 Jun 1. Available from: http://www.goldcopd.com/guidelines-global-strategyfor-diagnosis-management.html.57. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007; 370:786–796.58. Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008; 359:2355–2365.59. Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987; 106:196–204.60. Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006; 28:1245–1257.61. Almagro P, Cabrera FJ, Diez J, Boixeda R, Alonso Ortiz MB, Murio C, et al. Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD: the EPOC en Servicios de medicina interna (ESMI) study. Chest. 2012; 142:1126–1133.62. Fabbri LM, Luppi F, Beghe B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008; 31:204–212.63. Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005; (4):CD003566.64. Jabbour A, Macdonald PS, Keogh AM, Kotlyar E, Mellemkjaer S, Coleman CF, et al. Differences between beta-blockers in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized crossover trial. J Am Coll Cardiol. 2010; 55:1780–1787.65. Cao C, Wang R, Wang J, Bunjhoo H, Xu Y, Xiong W. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PLoS One. 2012; 7:e43892.66. Yamauchi Y, Hasegawa W, Yasunaga H, Sunohara M, Jo T, Takami K, et al. Paradoxical association between body mass index and in-hospital mortality in elderly patients with chronic obstructive pulmonary disease in Japan. Int J Chron Obstruct Pulmon Dis. 2014; 9:1337–1346.67. Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010; 363:1128–1138.68. Kim J, Lee JH, Kim Y, Kim K, Oh YM, Yoo KH, et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: a national cross-sectional cohort study. BMC Pulm Med. 2013; 13:51.69. Martinez CH, Okajima Y, Murray S, Washko GR, Martinez FJ, Silverman EK, et al. Impact of self-reported gastroesophageal reflux disease in subjects from COPDGene cohort. Respir Res. 2014; 15:62.70. Ferguson GT, Calverley PM, Anderson JA, Jenkins CR, Jones PW, Willits LR, et al. Prevalence and progression of osteoporosis in patients with COPD: results from the TOwards a Revolution in COPD Health study. Chest. 2009; 136:1456–1465.71. Madsen H, Brixen K, Hallas J. Screening, prevention and treatment of osteoporosis in patients with chronic obstructive pulmonary disease: a population-based database study. Clin Respir J. 2010; 4:22–29.72. Kunik ME, Roundy K, Veazey C, Souchek J, Richardson P, Wray NP, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005; 127:1205–1211.73. Chin HJ, Lee KH, Park CS, Son CW, Lee HY, Yu SK, et al. Prevalence and risk factors of depression in patients with chronic obstructive pulmonary disease. Tuberc Respir Dis. 2008; 65:191–197.74. Ryu YJ, Chun EM, Lee JH, Chang JH. Prevalence of depression and anxiety in outpatients with chronic airway lung disease. Korean J Intern Med. 2010; 25:51–57.75. Hwang YI, Lee YS, Oh YM, Lee SD, Park SW, Kim YS, et al. Prevalence of depression and its influence on health-related quality of life in COPD patients. Chest. 2011; 140:542A.76. Hwang YI, Kim HJ, Won WY, Joh JS, Oh YM, Jung KS, et al. Screening for depression in patients with chronic obstructive pulmonary disease: a systematic review. Korean J Med. 2012; 83:468–475.77. Choi HS, Choi JH, Park KH, Joo KJ, Ga H, Ko HJ, et al. Standardization of the Korean Version of Patient Health Questionnaire-9 as a screening instrument for major depressive disorder. J Korean Acad Fam Med. 2007; 28:114–119.78. Lim KH, Park YN, Kim DH, Shin IH, Lee WS, Kim JB. A preliminary study of the standardization of the Korean Version of the Patient Health Questionnaire-9. Korean J Health Promot Dis Prev. 2009; 9:275–281.79. Anthonisen NR, Connett JE, Enright PL, Manfreda J. Lung Health Study Research Group. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med. 2002; 166:333–339.80. National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365:395–409.81. Benfield T, Lange P, Vestbo J. COPD stage and risk of hospitalization for infectious disease. Chest. 2008; 134:46–53.82. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014; (3):CD010115.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Revised (2018) COPD Clinical Practice Guideline of the Korean Academy of Tuberculosis and Respiratory Disease: A Summary
- COPD Guideline Revised 2012: Contracted Version
- Management of Chronic Obstructive Pulmonary Disease
- Pathophysiology of Chronic Obstructive Pulmonary Disease
- General Concepts of Chronic Obstructive Pulmonary Disease