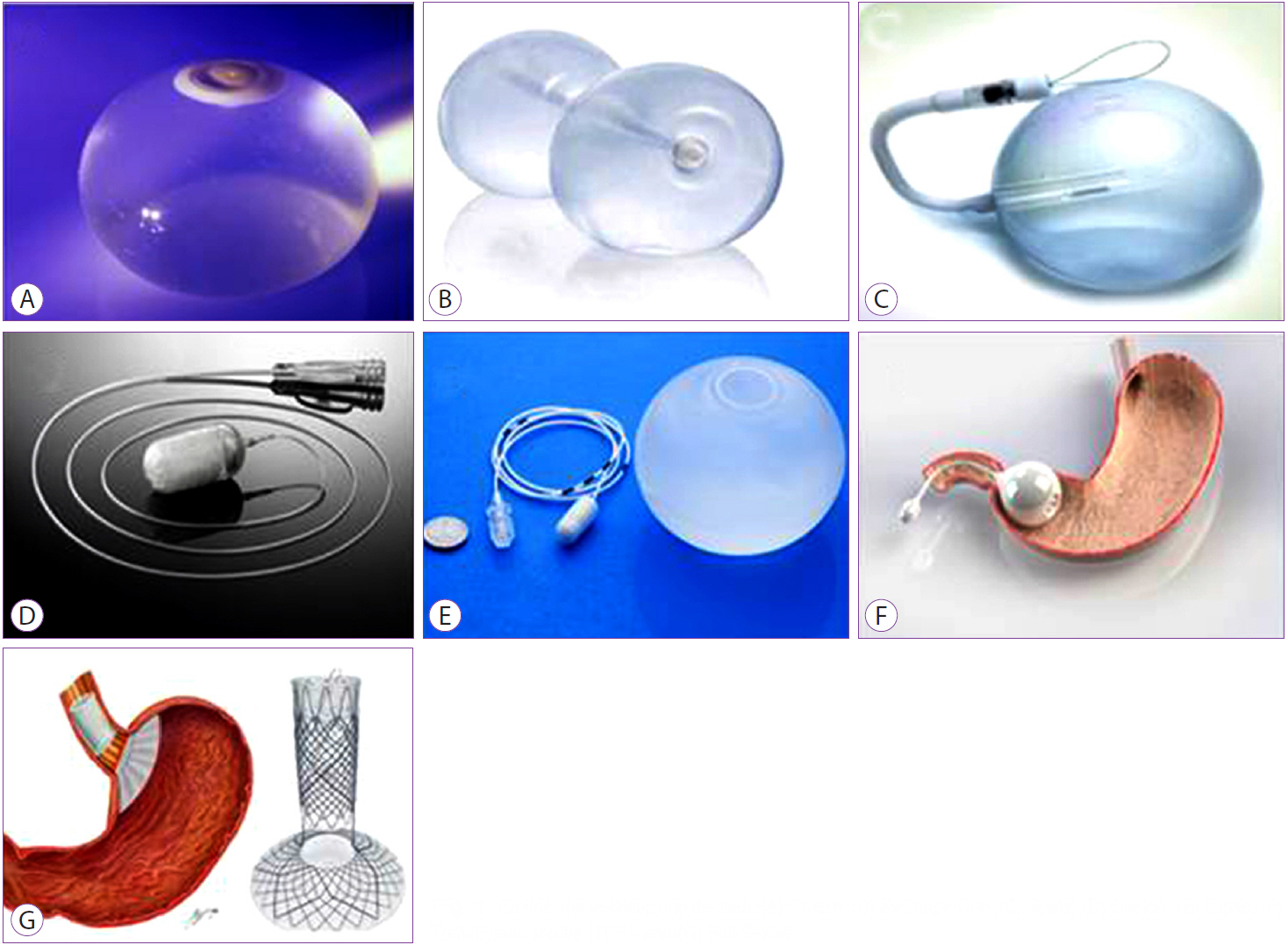
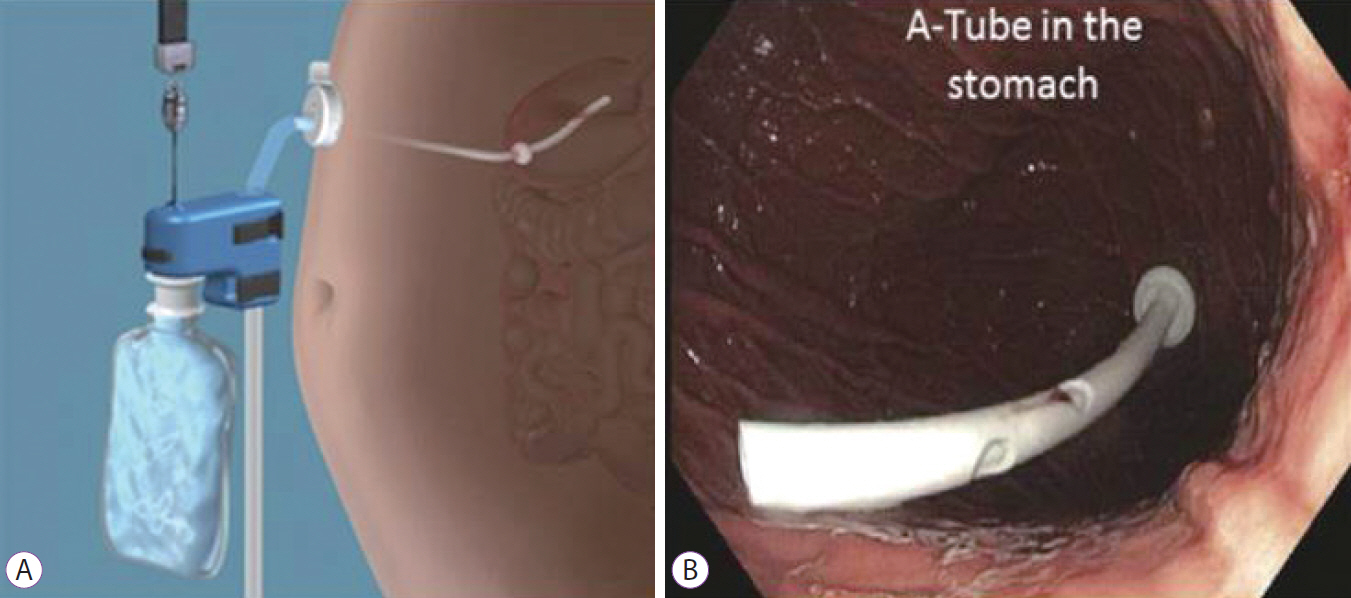
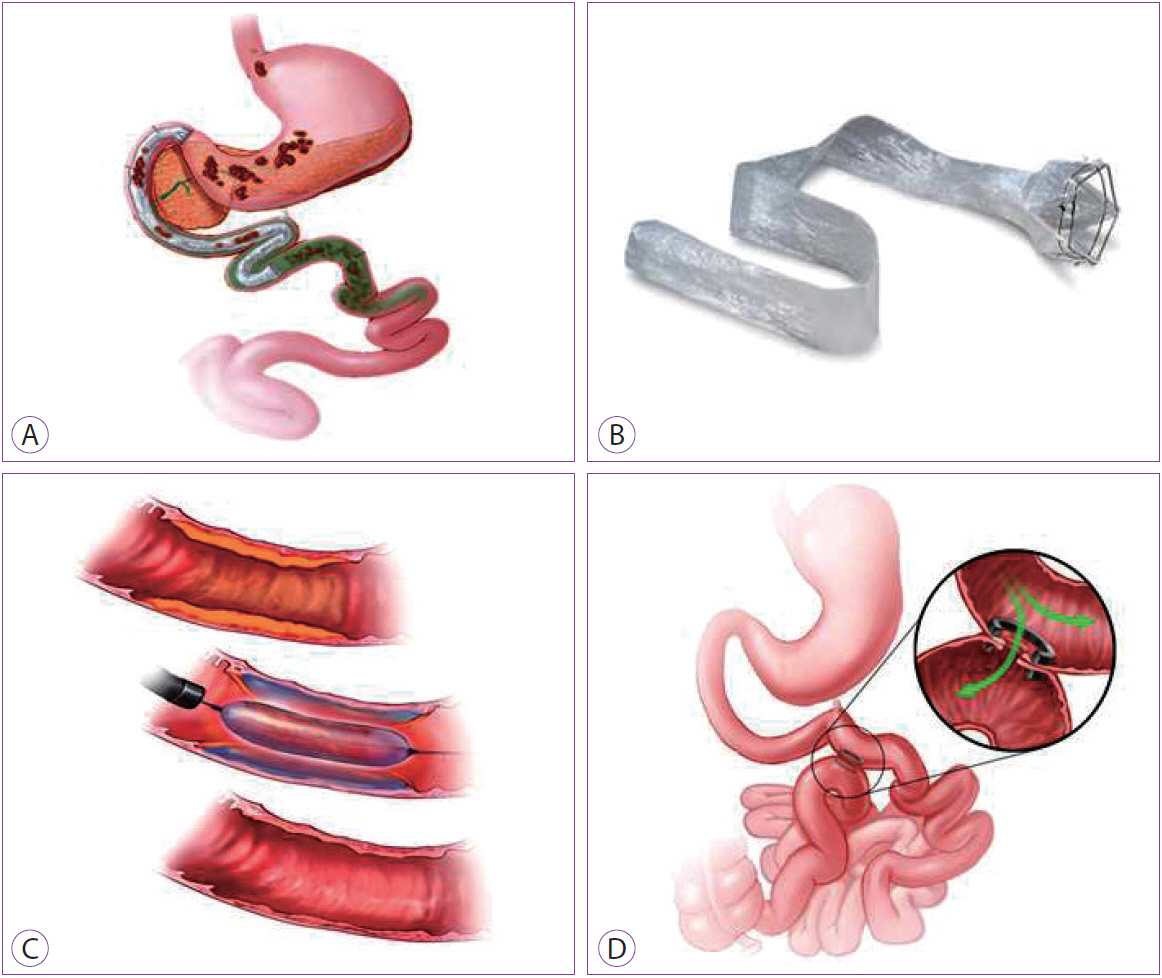
Clin Endosc.
2017 Jan;50(1):42-50. 10.5946/ce.2017.013.
Recent Clinical Results of Endoscopic Bariatric Therapies as an Obesity Intervention
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota, USA. AbuDayyeh.Barham@mayo.edu
- KMID: 2383489
- DOI: http://doi.org/10.5946/ce.2017.013
Abstract
- Despite advances in lifestyle interventions, anti-obesity medications, and metabolic surgery, the issue of health burden due to obesity continues to evolve. Interest in endoscopic bariatric techniques has increased over the years, as they have been shown to be efficacious, reversible, relatively safe, and cost effective. Further, these techniques offer a therapeutic window for some patients who may otherwise be unable to undergo bariatric surgery. This article aims to review the literature on the safety and efficacy of currently offered endoscopic bariatric techniques, as well as those that are in the pipeline of end-development and regulatory approval.
MeSH Terms
Figure
Cited by 1 articles
-
Various Intragastric Balloons Under Clinical Investigation
Seong Ji Choi, Hyuk Soon Choi
Clin Endosc. 2018;51(5):407-415. doi: 10.5946/ce.2018.140.
Reference
-
1. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010; 303:235–241.
Article2. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014; 384:766–781.3. Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006; 355:763–778.
Article4. Bellentani S, Marino M. Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD). Ann Hepatol. 2009; 8 Suppl 1:S4–S8.
Article5. Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005; 129:113–121.
Article6. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014; 311:74–86.7. Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009; 122:248–256. e5.
Article8. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012; 366:1567–1576.
Article9. Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009; 28:w822–w831.
Article10. Frühbeck G, Diez Caballero A, Gil MJ. Fundus functionality and ghrelin concentrations after bariatric surgery. N Engl J Med. 2004; 350:308–309.
Article11. Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am J Physiol. 1996; 271(3 Pt 2):R766–R769.
Article12. Wahlen CH, Bastens B, Herve J, et al. The bioenterics intragastric balloon (BIB): How to use it. Obes Surg. 2001; 11:524–527.
Article13. ASGE Bariatric Endoscopy Task Force and ASGE Technology Committee, Abu Dayyeh BK, Kumar N, et al. ASGE bariatric endoscopy task force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015; 82:425–438. e5.
Article14. Abu Dayyeh BK, Eaton LL, Woodman G. A randomized, multi-center study to evaluate the safety and effectiveness of an intragastric balloon as an adjunct to a behavioral modification program, in comparison with a behavioral modification program alone in the weight management of obese subjects. Gastrointest Endosc. 2015; 81(5 Suppl):AB147.15. FDA. ORBERA™ Intragastric balloon system-P140008 [Internet]. Silver Spring: U.S: Food and Drug Administration;c2015 [updated 2015 Aug 5; cited 2015 Dec 7]. Available from: http://www.fda.gov/medicaldevices/productsandmedicalprocedures/deviceapprovalsandclearances/recently-approveddevices/ucm457416.htm.16. Crea N, Pata G, Della Casa D, et al. Improvement of metabolic syndrome following intragastric balloon: 1 year follow-up analysis. Obes Surg. 2009; 19:1084–1088.
Article17. Fuller NR, Pearson S, Lau NS, et al. An intragastric balloon in the treatment of obese individuals with metabolic syndrome: a randomized controlled study. Obesity (Silver Spring). 2013; 21:1561–1570.
Article18. Lee YM, Low HC, Lim LG, et al. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: a pilot study. Gastrointest Endosc. 2012; 76:756–760.
Article19. Mui WL, Ng EK, Tsung BY, Lam CH, Yung MY. Impact on obesity-related illnesses and quality of life following intragastric balloon. Obes Surg. 2010; 20:1128–1132.
Article20. Kotzampassi K, Grosomanidis V, Papakostas P, Penna S, Eleftheriadis E. 500 intragastric balloons: what happens 5 years thereafter? Obes Surg. 2012; 22:896–903.
Article21. Gómez V, Woodman G, Abu Dayyeh BK. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: Results of a prospective study. Obesity (Silver Spring). 2016; 24:1849–1853.
Article22. Tai CM, Lin HY, Yen YC, et al. Effectiveness of intragastric balloon treatment for obese patients: one-year follow-up after balloon removal. Obes Surg. 2013; 23:2068–2074.
Article23. Dumonceau JM, François E, Hittelet A, Mehdi AI, Barea M, Deviere J. Single vs repeated treatment with the intragastric balloon: a 5-year weight loss study. Obes Surg. 2010; 20:692–697.
Article24. Genco A, Cipriano M, Bacci V, et al. Intragastric balloon followed by diet vs intragastric balloon followed by another balloon: a prospective study on 100 patients. Obes Surg. 2010; 20:1496–1500.
Article25. Genco A, Maselli R, Frangella F, et al. Effect of consecutive intragastric balloon (BIB®) plus diet versus single BIB® plus diet on eating disorders not otherwise specified (EDNOS) in obese patients. Obes Surg. 2013; 23:2075–2079.26. Lopez-Nava G, Rubio MA, Prados S, et al. BioEnterics® intragastric balloon (BIB®). Single ambulatory center Spanish experience with 714 consecutive patients treated with one or two consecutive balloons. Obes Surg. 2011; 21:5–9.27. FDA. ReShape integrated dual balloon system-P140012 [Internet]. Silver Spring: U.S: Food and Drug Administration;c2015 [updated 2015 Jul 28; cited 2015 Dec 7]. Available from: http://www.fda.gov/medicaldevices/productsandmedicalprocedures/deviceapprovalsandclearances/recently-approveddevices/ucm456293.htm.28. Ponce J, Woodman G, Swain J, et al. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015; 11:874–881.
Article29. Lopez-Nava G, Bautista-Castaño I, Jimenez-Baños A, Fernandez-Corbelle JP. Dual intragastric balloon: single ambulatory center Spanish experience with 60 patients in endoscopic weight loss management. Obes Surg. 2015; 25:2263–2267.
Article30. Ponce J, Quebbemann BB, Patterson EJ. Prospective, randomized, multicenter study evaluating safety and efficacy of intragastric dual-balloon in obesity. Surg Obes Relat Dis. 2013; 9:290–295.
Article31. Brooks J, Srivastava ED, Mathus-Vliegen EM. One-year adjustable intragastric balloons: results in 73 consecutive patients in the U.K. Obes Surg. 2014; 24:813–819.
Article32. Genco A, Dellepiane D, Baglio G, et al. Adjustable intragastric balloon vs non-adjustable intragastric balloon: case-control study on complications, tolerance, and efficacy. Obes Surg. 2013; 23:953–958.
Article33. FDA. Obalon balloon system-P160001 [Internet]. Silver Spring: U.S: Food and Drug Administration;c2016 [updated 2016 Sep 8; cited 2016 Dec 11]. Available from: http://www.fda.gov/medicaldevices/productsandmedicalprocedures/deviceapprovalsandclearances/recently-approveddevices/ucm520741.htm.34. Sullivan S, Swain JM, Woodman G, et al. The obalon swallowable 6-month balloon system is more effective than moderate intensity lifestyle therapy alone: results from a 6- month randomized sham controlled trial. Gastroenterology. 2016; 150(4 Suppl 1):S1267.35. Machytka E, Gaur S, Chuttani R, et al. Elipse, the first procedureless gastric balloon for weight loss: a prospective, observational, open-label, multicenter study. Endoscopy. 2016; Dec. 12. [Epub]. http://dx.doi.org/10.1055/s-0042-119296.
Article36. Marinos G, Eliades C, Raman Muthusamy V, Greenway F. Weight loss and improved quality of life with a nonsurgical endoscopic treatment for obesity: clinical results from a 3- and 6-month study. Surg Obes Relat Dis. 2014; 10:929–934.
Article37. Myall P. New endoscopic stent can lead to 100% EWL [Internet]. London: Bariatric News;c2012 [updated 2012 Jun 5; cited 2015 Dec 7]. Available from: http://www.bariatricnews.net/?q=node/102.38. Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc. 2013; 78:530–535.
Article39. Abu Dayyeh BK, Acosta A, Camilleri M, et al. Endoscopic sleeve gastroplasty alters gastric physiology and induces loss of body weight in obese individuals. Clin Gastroenterol Hepatol. 2017; 15:37–43. e1.40. Sharaiha RZ, Kedia P, Kumta N, et al. Initial experience with endoscopic sleeve gastroplasty: technical success and reproducibility in the bariatric population. Endoscopy. 2015; 47:164–166.
Article41. Lopez-Nava G, Galvao M, Bautista-Castaño I, Fernandez-Corbelle JP, Trell M. Endoscopic sleeve gastroplasty with 1-year follow-up: factors predictive of success. Endosc Int Open. 2016; 4:E222–E227.
Article42. Lopez-Nava G, Galvão MP, Bautista-Castaño I, Jimenez-Baños A, Fernandez-Corbelle JP. Endoscopic sleeve gastroplasty: how I do it? Obes Surg. 2015; 25:1534–1538.
Article43. Kumar N, Lopez-Nava G, Sahdala HNP, et al. Endoscopic sleeve gastroplasty: multicenter weight loss results. Gastroenterology. 2015; 148(4 Suppl 1):S–179.44. Lopez-Nava G, Sharaiha RZ, Neto MG, et al. Endoscopic sleeve gastroplasty for obesity: a multicenter study of 242 patients with 18 months follow-up. Gastroenterology. 2016; 150(4 Suppl 1):S26.45. Espinós JC, Turró R, Mata A, et al. Early experience with the Incisionless Operating Platform™ (IOP) for the treatment of obesity : the primary obesity surgery endolumenal (POSE) procedure. Obes Surg. 2013; 23:1375–1383.46. López-Nava G, Bautista-Castaño I, Jimenez A, de Grado T, Fernandez-Corbelle JP. The primary obesity surgery endolumenal (POSE) procedure: one-year patient weight loss and safety outcomes. Surg Obes Relat Dis. 2015; 11:861–865.
Article47. Miller K, Turró R, Greve JW, Bakker CM, Buchwald JN, Espinós JC. MILEPOST multicenter randomized controlled trial: 12-month weight loss and satiety outcomes after poseSM vs. medical therapy. Obes Surg. 2017; 27:310–322.48. Sullivan S, Swain JM, Woodman G, et al. 12 Month randomized sham controlled trial evaluating the safety and efficacy of targeted use of endoscopic suture anchors for primary obesity: the ESSENTIAL study. Gastroenterology. 2016; 150(4 Suppl 1):S25–S26.49. Abu Dayyeh B, Rajan E, Gostout hJ. Gastric endoscopic remodeling techniques. Tech Gastrointest Endosc. 2016; Dec. 6. [Epub]. http://dx.doi.org/10.1016/j.tgie.2016.12.004.
Article50. Huberty V, Ibrahim M, Hiernaux M, Chau A, Dugardeyn S, Deviere J. Safety and feasibility of an endoluminal-suturing device for endoscopic gastric reduction (with video). Gastrointest Endosc. 2016; Aug. 22. [Epub]. http://dx.doi.org/10.1016/j.gie.2016.08.007.
Article51. Thompson CC, Abu Dayyeh BK, Kushner R, et al. Percutaneous gastrostomy device for the treatment of class II and class III obesity: results of a randomized controlled trial. Am J Gastroenterol. 2016; Dec. 6. [Epub]. http://dx.doi.org/10.1038/ajg.2016.500.
Article52. Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006; 244:741–749.
Article53. Kaplan LM, Buse JB, Mullin C, et al. EndoBarrier therapy is associated with glycemic improvement, weight loss and safety issues in patients with obesity and type 2 diabetes on oral antihyperglycemic agents. In : Proceedings of the 76th scientific sessions; 2016 Jun 10-14; New Orleans, Louisiana, USA. Virginia. American diabetes association. 2016.54. Rajagopalan H, Cherrington AD, Thompson CC, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes: 6-month interim analysis from the first-in-human proof-of-concept study. Diabetes Care. 2016; 39:2254–2261.
Article