Endocrinol Metab.
2016 Dec;31(4):586-591. 10.3803/EnM.2016.31.4.586.
Molecular Diagnosis Using Residual Liquid-Based Cytology Materials for Patients with Nondiagnostic or Indeterminate Thyroid Nodules
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. kimwb@amc.seoul.kr
- 2Division of Endocrinology and Metabolism, Department of Oncology and Arnie Charbonneau Cancer Institute, Cummings School of Medicine, University of Calgary, Calgary, AB, Canada.
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2373079
- DOI: http://doi.org/10.3803/EnM.2016.31.4.586
Abstract
- BACKGROUND
Molecular analysis for common somatic mutations in thyroid cancer can improve diagnostic accuracy of fine-needle aspiration cytology (FNAC) in the nondiagnostic or indeterminate category of thyroid nodules. In this study, we evaluated the feasibility of molecular diagnosis from residual liquid-based cytology (LBC) material after cytological diagnosis.
METHODS
This prospective study enrolled 53 patients with thyroid nodules diagnosed as nondiagnostic, atypia of undetermined significance (AUS), or follicular lesion of undetermined significance (FLUS) after FNAC. DNAs and RNAs were isolated from residual LBC materials. BRAF(V600E) and RAS point mutations, PAX8/peroxisome proliferator-activated receptor γ (PPARγ), RET/PTC1, and RET/PTC3 rearrangements were evaluated by real-time polymerase chain reaction and pyrosequencing.
RESULTS
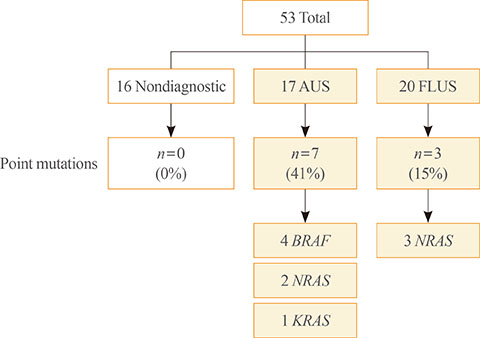
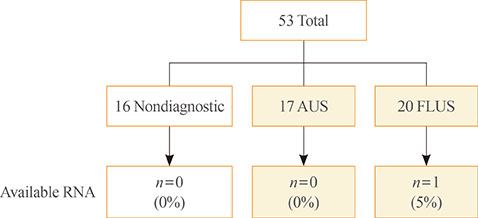
All DNAs from 53 residual LBC samples could be analysed and point mutations were detected in 10 samples (19%). In 17 AUS nodules, seven samples (41%) had point mutations including BRAF (n=4), NRAS (n=2), and KRAS (n=1). In 20 FLUS nodules, three samples (15%) had NRAS point mutations. RNA from only one FLUS nodule could be analysed for rearrangements and there was no abnormality.
CONCLUSION
Molecular analysis for BRAF and RAS mutations was feasible in residual LBC materials and might be useful for diagnosis of indeterminate thyroid nodules.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Comparison of Immunohistochemistry and Direct Sanger Sequencing for Detection of the BRAFV600E Mutation in Thyroid Neoplasm
Hye-Seon Oh, Hyemi Kwon, Suyeon Park, Mijin Kim, Min Ji Jeon, Tae Yong Kim, Young Kee Shong, Won Bae Kim, Jene Choi, Won Gu Kim, Dong Eun Song
Endocrinol Metab. 2018;33(1):62-69. doi: 10.3803/EnM.2018.33.1.62.
Reference
-
1. Cibas ES, Ali SZ. The bethesda system for reporting thyroid cytopathology. Thyroid. 2009; 19:1159–1165.2. Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008; 22:901–911.3. Vander JB, Gaston EA, Dawber TR. The significance of nontoxic thyroid nodules: final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med. 1968; 69:537–540.4. Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009; 39:699–706.5. Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013; 381:1058–1069.6. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.7. Crippa S, Mazzucchelli L, Cibas ES, Ali SZ. The Bethesda System for reporting thyroid fine-needle aspiration specimens. Am J Clin Pathol. 2010; 134:343–344.8. Hsiao SJ, Nikiforov YE. Molecular approaches to thyroid cancer diagnosis. Endocr Relat Cancer. 2014; 21:T301–T313.9. Song YS, Lim JA, Park YJ. Mutation profile of well-differentiated thyroid cancer in Asians. Endocrinol Metab (Seoul). 2015; 30:252–262.10. Rossi ED, Martini M, Capodimonti S, Straccia P, Cenci T, Lombardi CP, et al. Diagnostic and prognostic value of immunocytochemistry and BRAF mutation analysis on liquid-based biopsies of thyroid neoplasms suspicious for carcinoma. Eur J Endocrinol. 2013; 168:853–859.11. Eszlinger M, Krogdahl A, Münz S, Rehfeld C, Precht Jensen EM, Ferraz C, et al. Impact of molecular screening for point mutations and rearrangements in routine air-dried fine-needle aspiration samples of thyroid nodules. Thyroid. 2014; 24:305–313.12. Fadda G, Rossi ED. Liquid-based cytology in fine-needle aspiration biopsies of the thyroid gland. Acta Cytol. 2011; 55:389–400.13. Krane JF, Cibas ES, Alexander EK, Paschke R, Eszlinger M. Molecular analysis of residual ThinPrep material from thyroid FNAs increases diagnostic sensitivity. Cancer Cytopathol. 2015; 123:356–361.14. Kwon H, Kim WG, Choi YM, Jang EK, Jeon MJ, Song DE, et al. A cut-off value of basal serum calcitonin for detecting macroscopic medullary thyroid carcinoma. Clin Endocrinol (Oxf). 2015; 82:598–603.15. Rehfeld C, Munz S, Krogdahl A, Jensen EM, Siebolts U, Ferraz C, et al. Impact of different methodologies on the detection of point mutations in routine air-dried fine needle aspiration (FNA) smears. Horm Metab Res. 2013; 45:513–517.16. Afify A, Yu C, Hejazi N, Howell L. The diagnostic utility of cell blocks prepared from residual SurePath Pap material for detection of human papilloma virus. Appl Immunohistochem Mol Morphol. 2009; 17:108–114.17. Ohori NP, Singhal R, Nikiforova MN, Yip L, Schoedel KE, Coyne C, et al. BRAF mutation detection in indeterminate thyroid cytology specimens: underlying cytologic, molecular, and pathologic characteristics of papillary thyroid carcinoma. Cancer Cytopathol. 2013; 121:197–205.18. Musholt TJ, Fottner C, Weber MM, Eichhorn W, Pohlenz J, Musholt PB, et al. Detection of papillary thyroid carcinoma by analysis of BRAF and RET/PTC1 mutations in fine-needle aspiration biopsies of thyroid nodules. World J Surg. 2010; 34:2595–2603.19. Eszlinger M, Paschke R. Molecular fine-needle aspiration biopsy diagnosis of thyroid nodules by tumor specific mutations and gene expression patterns. Mol Cell Endocrinol. 2010; 322:29–37.20. Nikiforova MN, Nikiforov YE. Molecular diagnostics and predictors in thyroid cancer. Thyroid. 2009; 19:1351–1361.21. Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009; 94:2092–2098.22. Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007; 28:742–762.23. Hong AR, Lim JA, Kim TH, Choi HS, Yoo WS, Min HS, et al. The frequency and clinical implications of the BRAF (V600E) mutation in papillary thyroid cancer patients in Korea over the past two decades. Endocrinol Metab (Seoul). 2014; 29:505–513.24. Kim JG. Molecular pathogenesis and targeted therapies in well-differentiated thyroid carcinoma. Endocrinol Metab (Seoul). 2014; 29:211–216.25. Rossi ED, Zannoni GF, Moncelsi S, Stigliano E, Santeusanio G, Lombardi CP, et al. Application of liquid-based cytology to fine-needle aspiration biopsies of the thyroid gland. Front Endocrinol (Lausanne). 2012; 3:57.26. Yip L. Molecular diagnostic testing and the indeterminate thyroid nodule. Curr Opin Oncol. 2014; 26:8–13.27. Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003; 95:625–627.28. Xing M, Tufano RP, Tufaro AP, Basaria S, Ewertz M, Rosenbaum E, et al. Detection of BRAF mutation on fine needle aspiration biopsy specimens: a new diagnostic tool for papillary thyroid cancer. J Clin Endocrinol Metab. 2004; 89:2867–2872.29. Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011; 96:3390–3397.30. Chung KW, Yang SK, Lee GK, Kim EY, Kwon S, Lee SH, et al. Detection of BRAFV600E mutation on fine needle aspiration specimens of thyroid nodule refines cyto-pathology diagnosis, especially in BRAF600E mutation-prevalent area. Clin Endocrinol (Oxf). 2006; 65:660–666.31. Michael CW, McConnel J, Pecott J, Afify AM, Al-Khafaji B. Comparison of ThinPrep and TriPath PREP liquid-based preparations in nongynecologic specimens: a pilot study. Diagn Cytopathol. 2001; 25:177–184.32. Gilbert L, Oates E, Ratnam S. Stability of cervical specimens in SurePath medium for human papillomavirus testing with the Roche cobas 4800 assay. J Clin Microbiol. 2013; 51:3412–3414.33. Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 2002; 161:1961–1971.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- US Diagnosis for Thyroid Nodules with an Indeterminate Cytology
- Role of BRAF(V600E) Mutation Analysis for Thyroid Nodules Classified as Indeterminate on Ultrasonography
- Molecular Testing of Thyroid Indeterminate Nodules for Clinical Management Decision
- Thyroid Nodules with Nondiagnostic FNA Results: Role of Core Needle Biopsy
- Treatment of Thyroid Nodule