J Gastric Cancer.
2014 Jun;14(2):117-122.
Initial Clinical Experience with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in Signet-Ring Cell Gastric Cancer with Peritoneal Metastases
- Affiliations
-
- 1Department of General, Visceral and Transplant Surgery, University of Tubingen, Comprehensive Cancer Center, Tubingen, Germany. ingmar.koenigsrainer@med.uni-tuebingen.de
Abstract
- PURPOSE
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been shown to improve survival in select patients with gastric cancer and peritoneal metastases. It remains unclear, however, whether this multimodal treatment protocol is also beneficial for signet-ring cell gastric cancer (SRC) patients with peritoneal metastases.
MATERIALS AND METHODS
Clinical data of patients scheduled for upfront systemic chemotherapy consisting of 5-FU (2,600 mg/m2), folinic acid (200 mg/m2), docetaxel (50 mg/m2), and oxaliplatin (85 mg/m2) followed by CRS and HIPEC using cisplatin (50 mg/m2) at the Comprehensive Cancer Center, University Hospital Tubingen, Germany were retrospectively analyzed.
RESULTS
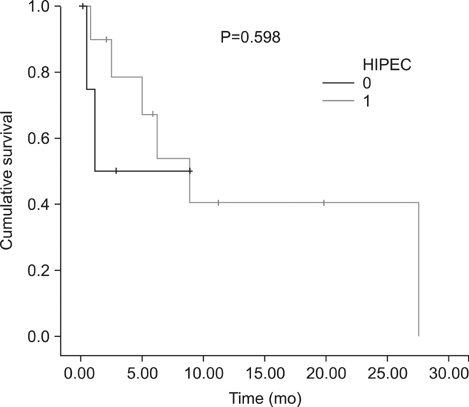
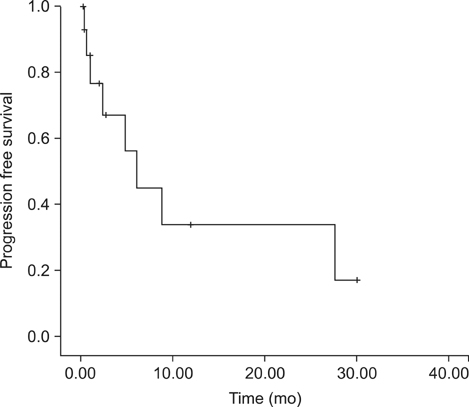
Eighteen consecutive patients for whom irresectability has been ruled out by a computed tomography scan were enrolled. However, complete cytoreduction could only be achieved in 72% of patients. When categorizing patients with respect to the completeness of cytoreduction, we found no difference between both groups considering tumor- or patient-related factors. The overall complication rate following complete cytoreduction and HIPEC was 46%. Within a median follow-up of 6.6 (0.5~31) months, the median survival for CRS and HIPEC patients was 8.9 months as opposed to 1.1 months for patients where complete cytoreduction could not be achieved. Following complete cytoreduction and HIPEC, progression-free survival was 6.2 months.
CONCLUSIONS
In SRC with peritoneal metastases, the prognosis appears to remain poor irrespective of complete CRS and HIPEC. Moreover, complete cytoreduction could not be achieved in a considerable percentage of patients. In SRC, CRS and HIPEC should be restricted to highly selective patients in order to avoid exploratory laparotomy.
MeSH Terms
Figure
Reference
-
1. Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003; 56:1–9.
Article2. Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999; 49:33–64.
Article3. Kim JY, Bae HS. A controlled clinical study of serosa-invasive gastric carcinoma patients who underwent surgery plus intraperitoneal hyperthermo-chemo-perfusion (IHCP). Gastric Cancer. 2001; 4:27–33.
Article4. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006; 24:4991–4997.
Article5. Yonemura Y, Endou Y, Shinbo M, Sasaki T, Hirano M, Mizumoto A, et al. Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: Selection for cytoreductive surgery. J Surg Oncol. 2009; 100:311–316.
Article6. Glehen O, Schreiber V, Cotte E, Sayag-Beaujard AC, Osinsky D, Freyer G, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg. 2004; 139:20–26.
Article7. Scaringi S, Kianmanesh R, Sabate JM, Facchiano E, Jouet P, Coffin B, et al. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol. 2008; 34:1246–1252.
Article8. Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, et al. Association Française de Chirurgie. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010; 17:2370–2377.
Article9. Hultman B, Lind P, Glimelius B, Sundbom M, Nygren P, Haglund U, et al. Phase II study of patients with peritoneal carcinomatosis from gastric cancer treated with preoperative systemic chemotherapy followed by peritonectomy and intraperitoneal chemotherapy. Acta Oncol. 2013; 52:824–830.
Article10. Hultman B, Lundkvist J, Glimelius B, Nygren P, Mahteme H. Costs and clinical outcome of neoadjuvant systemic chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from gastric cancer. Acta Oncol. 2012; 51:112–121.
Article11. Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009; 250:878–887.
Article12. Heger U, Blank S, Wiecha C, Langer R, Weichert W, Lordick F, et al. Is preoperative chemotherapy followed by surgery the appropriate treatment for signet ring cell containing adenocarcinomas of the esophagogastric junction and stomach? Ann Surg Oncol. 2014; 21:1739–1748.
Article13. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.14. Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996; 82:359–374.
Article15. Sugarbaker PH. Peritonectomy procedures. Surg Oncol Clin N Am. 2003; 12:703–727.
Article16. Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995; 221:29–42.
Article17. Sugarbaker PH. Surgical management of peritoneal carcinosis: diagnosis, prevention and treatment. Langenbecks Arch Chir. 1988; 373:189–196.
Article18. Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011; 18:1575–1581.
Article19. Yonemura Y, Elnemr A, Endou Y, Ishibashi H, Mizumoto A, Miura M, et al. Effects of neoadjuvant intraperitoneal/systemic chemotherapy (bidirectional chemotherapy) for the treatment of patients with peritoneal metastasis from gastric cancer. Int J Surg Oncol. 2012; 2012:148420.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for ovarian cancer
- Advances in the Treatment of Colorectal Cancer with Peritoneal Metastases: A Focus on Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy
- Recent Update on the Treatment of Colorectal Peritoneal Metastasis: A Surgical Perspective
- Treatment of Peritoneal Carcinomatosis from Colorectal Cancer
- Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy as Treatment Options for Peritoneal Metastasis of Advanced Gastric Cancer