Int J Stem Cells.
2016 Nov;9(2):239-249. 10.15283/ijsc16011.
Intramuscular Transplantation of Allogeneic Mesenchymal Stromal Cells Derived from Equine Umbilical Cord
- Affiliations
-
- 1Department of Animal Reproduction and Veterinary Radiology, College of Veterinary Medicine and Animal Science, São Paulo State University, UNESP, Botucatu, SP, Brazil. leandromvet@hotmail.com
- 2Department of Veterinary Pathology, College of Veterinary Medicine and Animal Science, São Paulo State University, UNESP, Botucatu, SP, Brazil.
- KMID: 2362518
- DOI: http://doi.org/10.15283/ijsc16011
Abstract
- BACKGROUND AND OBJECTIVES
Mesenchymal stromal cells (MSCs) have great therapeutic potential, particularly in the process of tissue repair and immunomodulation through the secretion of biomolecules. Thus, the aim of this study was to evaluate the hypothesis that intramuscular transplantation of allogeneic MSCs obtained from equine umbilical cord (UC-MSCs) is safe, demonstrating that this is a suitable source of stem cells for therapeutic use.
METHODS AND RESULTS
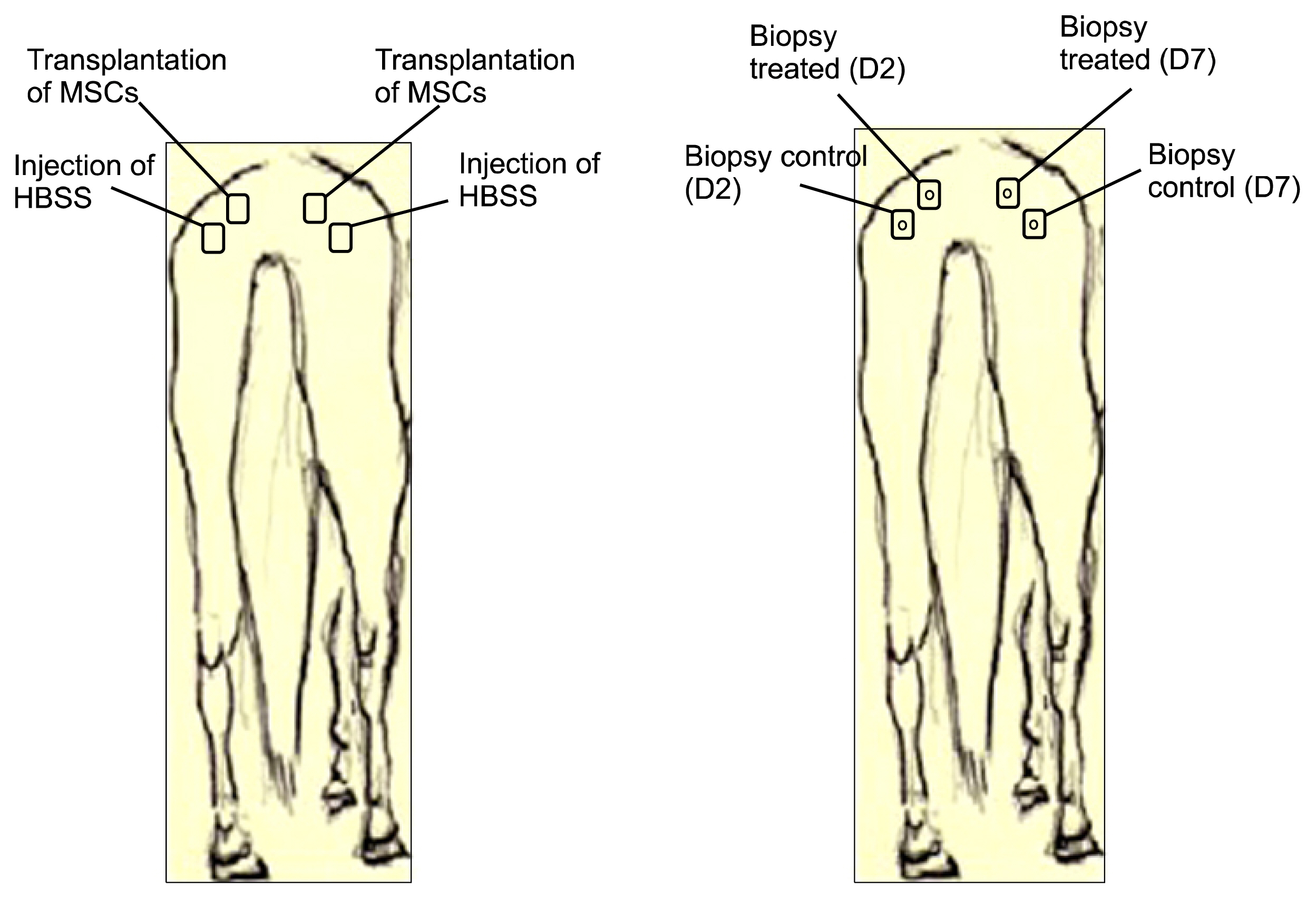
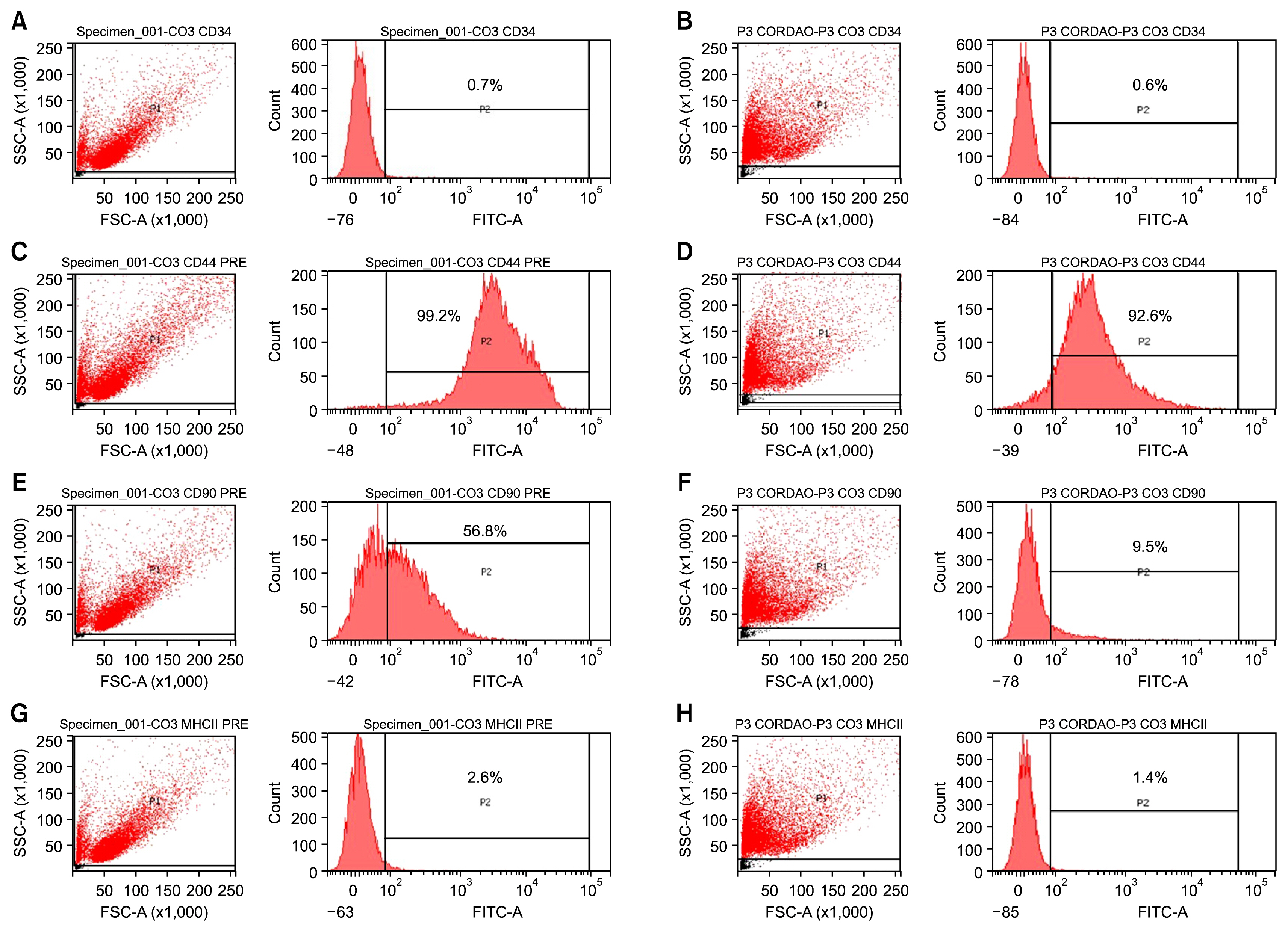
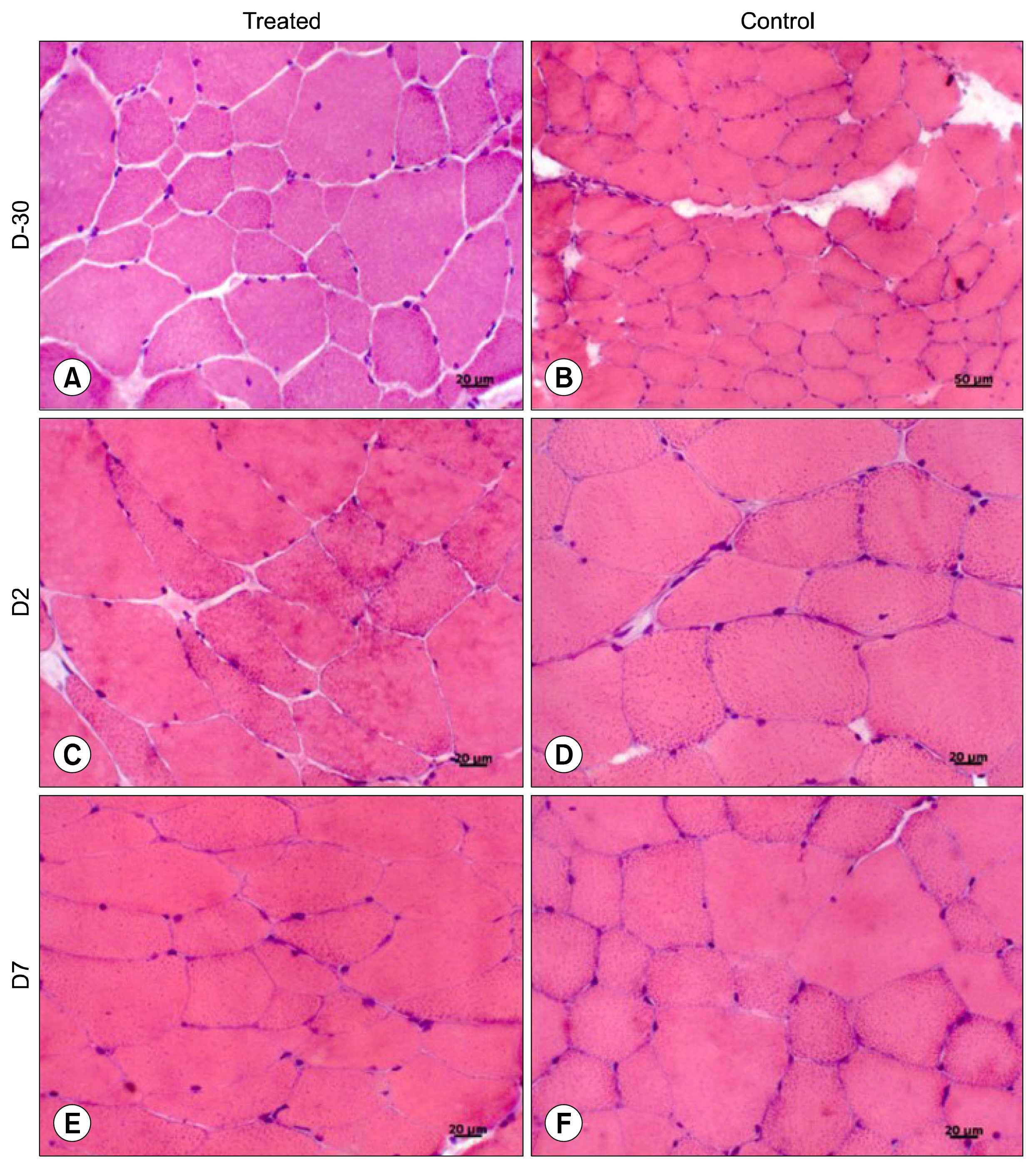
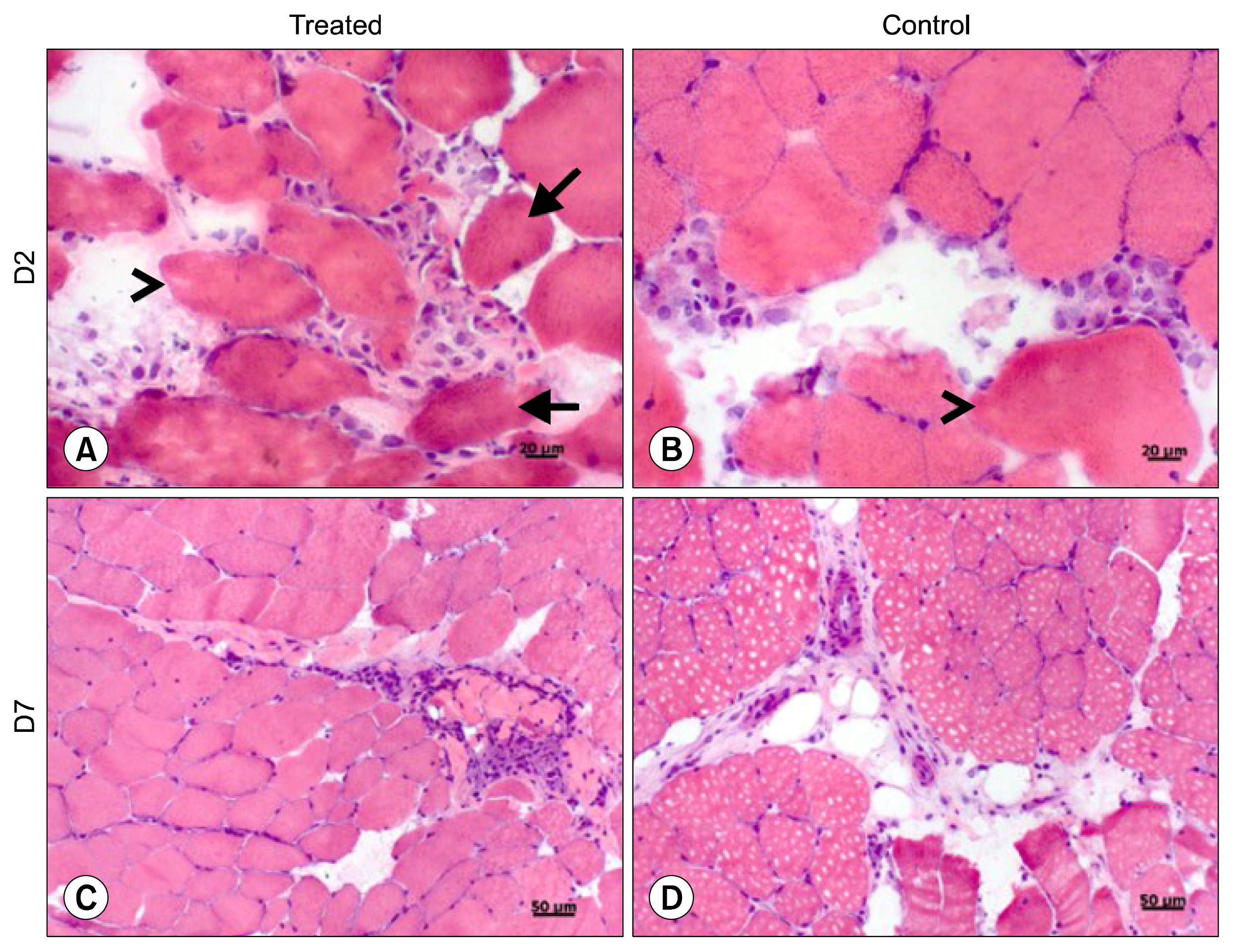
For this, UC-MSCs were cultured, characterized and cryopreserved for future transplantation in six healthy mares. On day 0, transplantation of three million UC-MSCs diluted in Hank's Balanced Solution (HBSS) was performed on right and left sides of the rump muscle. As a control, HBSS injections were performed caudally in the same muscle. Muscle biopsies were obtained as a control 30 days before transplantation (D-30). The biopsies were collected again on day 2 (left side) and day 7 (right side) post transplantation and examined histologically. All procedures were preceded by ultrasound examination and blood sampling. Hematologic evaluation remained within normal limits and no differences were observed between time points (p>0.05). Ultrasound examination was suggestive of inflammation 48 hours after transplantation in both groups (control and treated). At histological evaluation it was found only discrete inflammation signals between D-30×D2 (p<0.05) in the treated group, without differences (p> 0.05) between the groups at different time points.
CONCLUSIONS
Equine UC-MSCs under the experimental conditions did not promote severe inflammation that causes tissue damage or lead to its rejection by the host organism and therefore has a good potential for clinical use.
MeSH Terms
Figure
Reference
-
References
1. Voswinkel J, Francois S, Gorin NC, Chapel A. Gastro-intestinal autoimmunity: preclinical experiences and successful therapy of fistulizing bowel diseases and gut Graft versus host disease by mesenchymal stromal cells. Immunol Res. 2013; 56:241–248. DOI: 10.1007/s12026-013-8397-8. PMID: 23564182.
Article2. Durando MM, Zarucco L, Schaer TP, Ross M, Reef VB. Pneumopericardium in a horse secondary to sternal bone marrow aspiration. Equine Vet Educ. 2010; 18:75–79. DOI: 10.1111/j.2042-3292.2006.tb00419.x.
Article3. Paterson YZ, Rash N, Garvican ER, Paillot R, Guest DJ. Equine mesenchymal stromal cells and embryo-derived stem cells are immune privileged in vitro. Stem Cell Res Ther. 2014; 5:90. DOI: 10.1186/scrt479. PMID: 25080326. PMCID: 4247727.
Article4. Dyson SJ. Are mesenchymal progenitor cells set to revolutionise management of musculoskeletal injuries in the horse? Vet J. 2013; 197:533–534. DOI: 10.1016/j.tvjl.2013.05.018. PMID: 23778255.
Article5. Carrade DD, Affolter VK, Outerbridge CA, Watson JL, Galuppo LD, Buerchler S, Kumar V, Walker NJ, Borjesson DL. Intradermal injections of equine allogeneic umbilical cord-derived mesenchymal stem cells are well tolerated and do not elicit immediate or delayed hypersensitivity reactions. Cytotherapy. 2011; 13:1180–1192. DOI: 10.3109/14653249.2011.602338. PMID: 21899391.
Article6. Carrade DD, Owens SD, Galuppo LD, Vidal MA, Ferraro GL, Librach F, Buerchler S, Friedman MS, Walker NJ, Borjesson DL. Clinicopathologic findings following intra-articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy. 2011; 13:419–430. DOI: 10.3109/14653249.2010.536213.
Article7. Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009; 11:377–391. DOI: 10.1080/14653240903080367. PMID: 19568970.
Article8. Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013; 2013:130763. DOI: 10.1155/2013/130763. PMID: 24194766. PMCID: 3806396.
Article9. Pigott JH, Ishihara A, Wellman ML, Russell DS, Bertone AL. Inflammatory effects of autologous, genetically modified autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet Comp Orthop Traumatol. 2013; 26:453–460. DOI: 10.3415/VCOT-13-01-0008. PMID: 24080668.
Article10. Maia L, da Cruz Landim-Alvarenga F, Taffarel MO, de Moraes CN, Machado GF, Melo GD, Amorim RM. Feasibility and safety of intrathecal transplantation of autologous bone marrow mesenchymal stem cells in horses. BMC Vet Res. 2015; 11:63. DOI: 10.1186/s12917-015-0361-5. PMID: 25879519. PMCID: 4369105.
Article11. Lovati AB, Corradetti B, Lange Consiglio A, Recordati C, Bonacina E, Bizzaro D, Cremonesi F. Comparison of equine bone marrow-, umbilical cord matrix and amniotic fluid-derived progenitor cells. Vet Res Commun. 2011; 35:103–121. DOI: 10.1007/s11259-010-9457-3. PMID: 21193959.
Article12. Smith RK, Garvican ER, Fortier LA. The current ‘state of play’ of regenerative medicine in horses: what the horse can tell the human. Regen Med. 2014; 9:673–685. DOI: 10.2217/rme.14.42.
Article13. Maia L, Landim-Alvarenga FC, Da Mota LS, De Assis Golim M, Laufer-Amorim R, De Vita B, Barberini DJ, Listoni AJ, De Moraes CN, Heckler MC, Amorim RM. Immunophenotypic, immunocytochemistry, ultrastructural, and cytogenetic characterization of mesenchymal stem cells from equine bone marrow. Microsc Res Tech. 2013; 76:618–624. DOI: 10.1002/jemt.22208. PMID: 23533133.
Article14. Mensing N, Gasse H, Hambruch N, Haeger JD, Pfarrer C, Staszyk C. Isolation and characterization of multipotent mesenchymal stromal cells from the gingiva and the periodontal ligament of the horse. BMC Vet Res. 2011; 7:42. DOI: 10.1186/1746-6148-7-42. PMID: 21810270. PMCID: 3161857.
Article15. Jain NC. Essentials of veterinary hematology. Philadelphia: Lea & Febiger;1993. p. 417.16. Meyer DJ, Harvey JW. Veterinary laboratory medicine: interpretation & diagnosis. 2nd ed. Philadelphia: Sauders;2004. p. 351.17. Padilha FGF, Suma R, Amorim RM. Avaliação prática da biópsia muscular percutânea por agulha em equinos. Hora Vet. 2008; 28:29–32.18. Brasileiro JL, Fagundes DJ, Miiji LON, Oshima CTF, Teruya R, Marks G, Inouye CM, Santos MA. Isquemia e reperfusão de músculo sóleo de ratos sob ação da pentoxifilina. J Vasc Bras. 2007; 6:50–63. DOI: 10.1590/S1677-54492007000100008.
Article19. Corradetti B, Lange-Consiglio A, Barucca M, Cremonesi F, Bizzaro D. Size-sieved subpopulations of mesenchymal stem cells from intervascular and perivascular equine umbilical cord matrix. Cell Prolif. 2011; 44:330–342. DOI: 10.1111/j.1365-2184.2011.00759.x. PMID: 21645152.
Article20. Iacono E, Brunori L, Pirrone A, Pagliaro PP, Ricci F, Tazzari PL, Merlo B. Isolation, characterization and differentiation of mesenchymal stem cells from amniotic fluid, umbilical cord blood and Wharton’s jelly in the horse. Reproduction. 2012; 143:455–468. DOI: 10.1530/REP-10-0408. PMID: 22274885.
Article21. De Schauwer C, Piepers S, Van de Walle GR, Demeyere K, Hoogewijs MK, Govaere JL, Braeckmans K, Van Soom A, Meyer E. In search for cross-reactivity to immunophenotype equine mesenchymal stromal cells by multicolor flow cytometry. Cytometry A. 2012; 81:312–323. DOI: 10.1002/cyto.a.22026. PMID: 22411893.
Article22. Ranera B, Lyahyai J, Romero A, Vázquez FJ, Remacha AR, Bernal ML, Zaragoza P, Rodellar C, Martín-Burriel I. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet Immunol Immunopathol. 2011; 144:147–154. DOI: 10.1016/j.vetimm.2011.06.033. PMID: 21782255.
Article23. Schnabel LV, Pezzanite LM, Antczak DF, Felippe MJ, Fortier LA. Equine bone marrow-derived mesenchymal stromal cells are heterogeneous in MHC class II expression and capable of inciting an immune response in vitro. Stem Cell Res Ther. 2014; 5:13. DOI: 10.1186/scrt402. PMID: 24461709. PMCID: 4055004.
Article24. Barberini DJ, Freitas NP, Magnoni MS, Maia L, Listoni AJ, Heckler MC, Sudano MJ, Golim MA, da Cruz Landim-Alvarenga F, Amorim RM. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: immunophenotypic characterization and differentiation potential. Stem Cell Res Ther. 2014; 5:25. DOI: 10.1186/scrt414. PMID: 24559797. PMCID: 4055040.
Article25. Lew HL, Chen CP, Wang TG, Chew KT. Introduction to musculoskeletal diagnostic ultrasound: examination of the upper limb. Am J Phys Med Rehabil. 2007; 86:310–321. DOI: 10.1097/PHM.0b013e31803839ac. PMID: 17413545.26. Peetrons P. Ultrasound of muscles. Eur Radiol. 2002; 12:35–43. DOI: 10.1007/s00330-001-1164-6. PMID: 11868072.
Article27. Kol A, Wood JA, Carrade Holt DD, Gillette JA, Bohannon-Worsley LK, Puchalski SM, Walker NJ, Clark KC, Watson JL, Borjesson DL. Multiple intravenous injections of allogeneic equine mesenchymal stem cells do not induce a systemic inflammatory response but do alter lymphocyte subsets in healthy horses. Stem Cell Res Ther. 2015; 6:73. DOI: 10.1186/s13287-015-0050-0. PMID: 25888916. PMCID: 4446064.
Article28. Gala K, Burdzińska A, Idziak M, Wilczek E, Pa̧czek L. Transplantation of mesenchymal stem cells into the skeletal muscle induces cytokine generation. Cytokine. 2013; 64:243–250. DOI: 10.1016/j.cyto.2013.06.314. PMID: 23859809.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Percutaneous transplantation of human umbilical cord-derived mesenchymal stem cells in a dog suspected to have fibrocartilaginous embolic myelopathy
- Characterization and clinical application of mesenchymal stem cells from equine umbilical cord blood
- Use of Cord Blood Stem Cells in Cell Therapy
- Difference in HLA-DR Expression of Human Umbilical Cord Blood Derived Mesenchymal Stem Cells after Tri-lineage Differentiation
- Immunologic properties of differentiated and undifferentiated mesenchymal stem cells derived from umbilical cord blood