Nutr Res Pract.
2015 Dec;9(6):606-612. 10.4162/nrp.2015.9.6.606.
Antiobesity effects of the water-soluble fraction of the ethanol extract of Smilax china L. leaf in 3T3-L1 adipocytes
- Affiliations
-
- 1Research and Development Division, Korean Promotion Institute for Traditional Medicine Industry, Gyeongsan, 712-260, Korea.
- 2Department of Bio-Health Technology, College of Biomedical Science, Kangwon National University, 1 Gangwondaehak-gil, Chuncheon, Gangwon 200-701, Korea. mchoe@kangwon.ac.kr
- 3Well-Being Bioproducts R&D Regional Innovation Center, Kangwon National University, 1 Gangwondaehak-gil, Chuncheon, Gangwon 200-701, Korea.
- KMID: 2313884
- DOI: http://doi.org/10.4162/nrp.2015.9.6.606
Abstract
- BACKGROUND/OBJECTIVES
Several medicinal properties of Smilax china L. have been studied including antioxidant, anti-inflammatory, and anti-cancer effects. However, the antiobesity activity and mechanism by which the water-soluble fraction of this plant mediates its effects are not clear. In the present study, we investigated the lipolytic actions of the water-soluble fraction of Smilax china L. leaf ethanol extract (wsSCLE) in 3T3-L1 adipocytes.
MATERIALS/METHODS
The wsSCLE was identified by measuring the total polyphenol and flavonoid content. The wsSCLE was evaluated for its effects on cell viability, lipid accumulation, glycerol, and cyclic adenosine monophosphate (cAMP) contents. In addition, western blot analysis was used to evaluate the effects on protein kinase A (PKA), PKA substrates (PKAs), and hormone-sensitive lipase (HSL). For the lipid accumulation assay, 3T3-L1 adipocytes were treated with different doses of wsSCLE for 9 days starting 2 days post-confluence. In other cell experiments, mature 3T3-L1 adipocytes were treated for 24 h with wsSCLE.
RESULTS
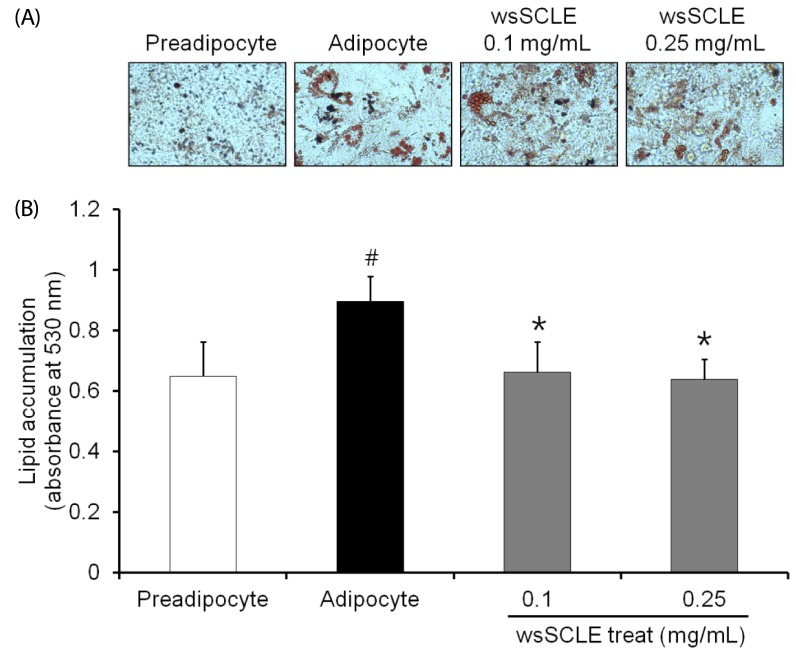
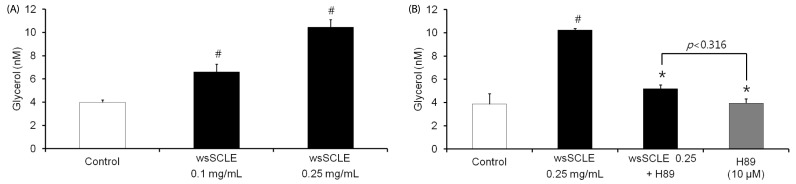
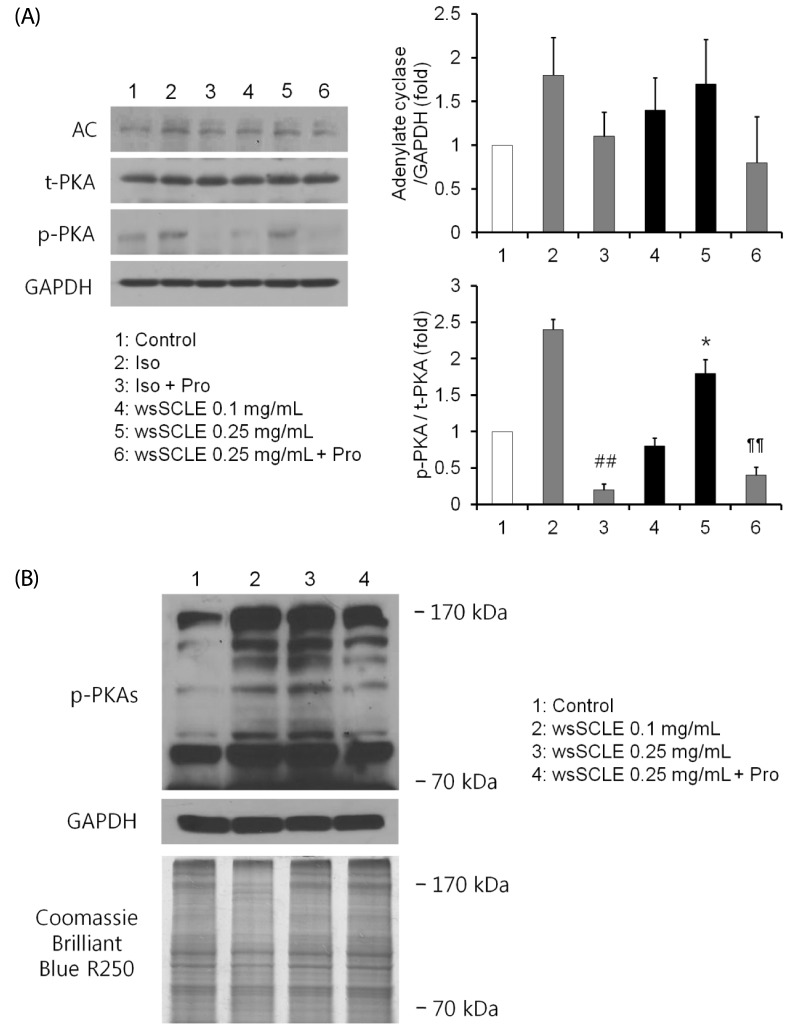
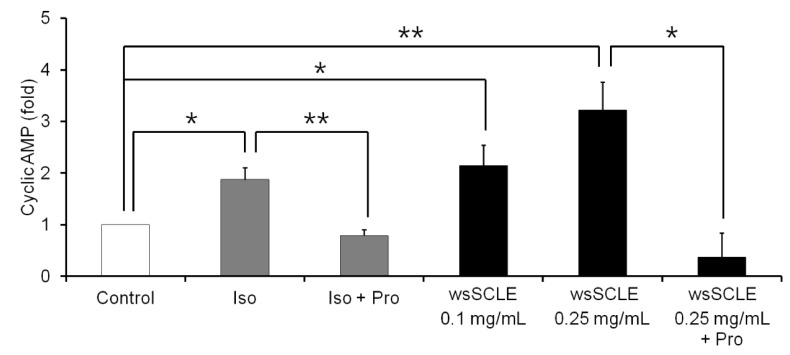
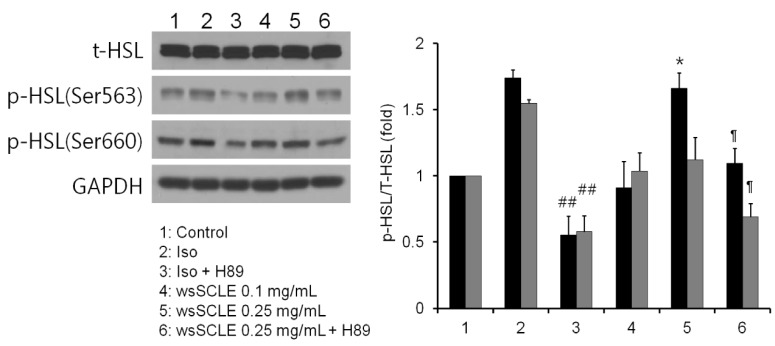
Results showed that treatment with wsSCLE at 0.05, 0.1, and 0.25 mg/mL had no effect on cell morphology and viability. Without evidence of toxicity, wsSCLE treatment decreased lipid accumulation compared with the untreated adipocyte controls as shown by the lower absorbance of Oil Red O stain. The wsSCLE significantly induced glycerol release and cAMP production in mature 3T3-L1 cells. Furthermore, protein levels of phosphorylated PKA, PKAs, and HSL significantly increased following wsSCLE treatment.
CONCLUSION
These results demonstrate that the potential antiobesity activity of wsSCLE is at least in part due to the stimulation of cAMP-PKA-HSL signaling. In addition, the wsSCLE-stimulated lipolysis induced by the signaling is mediated via activation of the beta-adrenergic receptor.
MeSH Terms
Figure
Reference
-
1. McMorrow AM, Connaughton RM, Lithander FE, Roche HM. Adipose tissue dysregulation and metabolic consequences in childhood and adolescent obesity: potential impact of dietary fat quality. Proc Nutr Soc. 2015; 74:67–82. PMID: 25497038.
Article3. Okabe Y, Shimada T, Horikawa T, Kinoshita K, Koyama K, Ichinose K, Aburada M, Takahashi K. Suppression of adipocyte hypertrophy by polymethoxyflavonoids isolated from Kaempferia parviflora. Phytomedicine. 2014; 21:800–806. PMID: 24629599.
Article4. Chaves VE, Frasson D, Kawashita NH. Several agents and pathways regulate lipolysis in adipocytes. Biochimie. 2011; 93:1631–1640. PMID: 21658426.
Article5. Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003; 31:1120–1124. PMID: 14641008.
Article6. Okumura T, Harada K, Oue K, Zhang J, Asano S, Hayashiuchi M, Mizokami A, Tanaka H, Irifune M, Kamata N, Hirata M, Kanematsu T. Phospholipase C-related catalytically inactive protein (PRIP) regulates lipolysis in adipose tissue by modulating the phosphorylation of hormone-sensitive lipase. PLoS One. 2014; 9:e100559. PMID: 24945349.
Article7. Kim SO, Sakchaisri K, Asami Y, Ryoo IJ, Choo SJ, Yoo ID, Soung NK, Kim YS, Jang JH, Kim BY, Ahn JS. Illudins C2 and C3 stimulate lipolysis in 3T3-L1 adipocytes and suppress adipogenesis in 3T3-L1 preadipocytes. J Nat Prod. 2014; 77:744–750. PMID: 24597820.
Article8. Jaworski K, Sarkadi-Nagy E, Duncan RE, Ahmadian M, Sul HS. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am J Physiol Gastrointest Liver Physiol. 2007; 293:G1–G4. PMID: 17218471.9. Chaput JP, St-Pierre S, Tremblay A. Currently available drugs for the treatment of obesity: Sibutramine and orlistat. Mini Rev Med Chem. 2007; 7:3–10. PMID: 17266632.
Article10. Collins P, Williams G. Drug treatment of obesity: from past failures to future successes? Br J Clin Pharmacol. 2001; 51:13–25. PMID: 11167661.
Article11. Li M, Cheung BM. Pharmacotherapy for obesity. Br J Clin Pharmacol. 2009; 68:804–810. PMID: 20002075.
Article12. Vickers SP, Cheetham SC, Headland KR, Dickinson K, Grempler R, Mayoux E, Mark M, Klein T. Combination of the sodium-glucose cotransporter-2 inhibitor empagliflozin with orlistat or sibutramine further improves the body-weight reduction and glucose homeostasis of obese rats fed a cafeteria diet. Diabetes Metab Syndr Obes. 2014; 7:265–275. PMID: 25061325.
Article13. Kumar P, Bhandari U, Jamadagni S. Fenugreek seed extract inhibit fat accumulation and ameliorates dyslipidemia in high fat diet-induced obese rats. Biomed Res Int. 2014; 2014:606021. PMID: 24868532.
Article14. Cha BC, Lee EH. Antioxidant activities of flavonoids from the leaves of Smilax china linne. Korean J Pharmacogn. 2007; 38:31–36.15. Kim SH, Ahn JH, Jeong JY, Kim SB, Jo YH, Hwang BY, Lee MK. Tyrosinase inhibitory phenolic constituents of Smilax china leaves. Korean J Pharmacogn. 2013; 44:220–223.16. Aguirre L, Fernández-Quintela A, Arias N, Portillo MP. Resveratrol: anti-obesity mechanisms of action. Molecules. 2014; 19:18632–18655. PMID: 25405284.
Article17. Park UH, Jeong JC, Jang JS, Sung MR, Youn H, Lee SJ, Kim EJ, Um SJ. Negative regulation of adipogenesis by kaempferol, a component of Rhizoma Polygonati falcatum in 3T3-L1 cells. Biol Pharm Bull. 2012; 35:1525–1533. PMID: 22975504.18. Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, Bapat P, Kwun I, Shen CL. Novel insights of dietary polyphenols and obesity. J Nutr Biochem. 2014; 25:1–18. PMID: 24314860.
Article19. Sakagami Y. Inhibitory effect of the extract of Prunus mume Sieb. et Zucc. on vero-toxin production by enterohemorrhagic Escherichia coli O157 : H7. Biocontrol Sci. 2001; 6:53–56.20. Kim KK, Kang YH, Kim DJ, Kim TW, Choe M. Comparison of antioxidant, α-glucosidase inhibition and anti-inflammatory activities of the leaf and root extracts of Smilax china L. J Nutr Health. 2013; 46:315–323.21. Moreno MI, Isla MI, Sampietro AR, Vattuone MA. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 2000; 71:109–114. PMID: 10904153.
Article22. Yadav D, Chaudhary AA, Garg V, Anwar MF, Rahman MM, Jamil SS, Khan HA, Asif M. In vitro toxicity and antidiabetic activity of a newly developed polyherbal formulation (MAC-ST/001) in streptozotocin-induced diabetic Wistar rats. Protoplasma. 2013; 250:741–749. PMID: 23053765.
Article23. Oh SD, Kim M, Min BI, Choi GS, Kim SK, Bae H, Kang C, Kim DG, Park BJ, Kim CK. Effect of Achyranthes bidentata Blume on 3T3-L1 adipogenesis and rats fed with a high-fat diet. Evid Based Complement Alternat Med. 2014; 2014:158018. PMID: 24963319.24. Jin TY, Park JR, Kim JH. Electron donating abilities, nitrite scavenging effects and antimicrobial activities of Smilax china leaf. J Korean Soc Food Sci Nutr. 2004; 33:621–625.25. Lee SY, Kim JH, Park JM, Lee IC, Lee JY. Antioxidant activity and inhibition activity against α-amylase and α-glucosidase of Smilax china L. Korean J Food Preserv. 2014; 21:254–263.
Article26. Park HS, Kim GH, Shim SM. Different effect of methanol extracts and bioaccessible fraction of Smilax china on triglyceride accumulation in adipocytes. J Food Biochem. 2014; 38:1–5.
Article27. Brasaemle DL, Levin DM, Adler-Wailes DC, Londos C. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim Biophys Acta. 2000; 1483:251–262. PMID: 10634941.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Study on antioxidative, antidiabetic and antiobesity activity of solvent fractions of smilax china L. leaf extract
- Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- Nelumbo nucifera Leaf Extract Regulates Lipid Metabolism and Differentiation in 3T3-L1 Adipocytes and db/db Mice
- Soluble extract of soybean fermented with Aspergillus oryzae GB107 inhibits fat accumulation in cultured 3T3-L1 adipocytes
- Anti-adipogenic effect of mulberry leaf ethanol extract in 3T3-L1 adipocytes