Nutr Res Pract.
2007 Dec;1(4):279-284.
Protective actions of Rubus coreanus ethanol extract on collagenous extracellular matrix in ultraviolet-B irradiation-induced human dermal fibroblasts
- Affiliations
-
- 1Department of Food and Nutrition and Korean Institute of Nutrition, Hallym University, Chuncheon, Korea. yhkang@hallym.ac.kr
- 2Department of Food and Nutrition and Korean Institute of Nutrition; Regional Innovation Center, Hallym University, Chuncheon, Korea.
Abstract
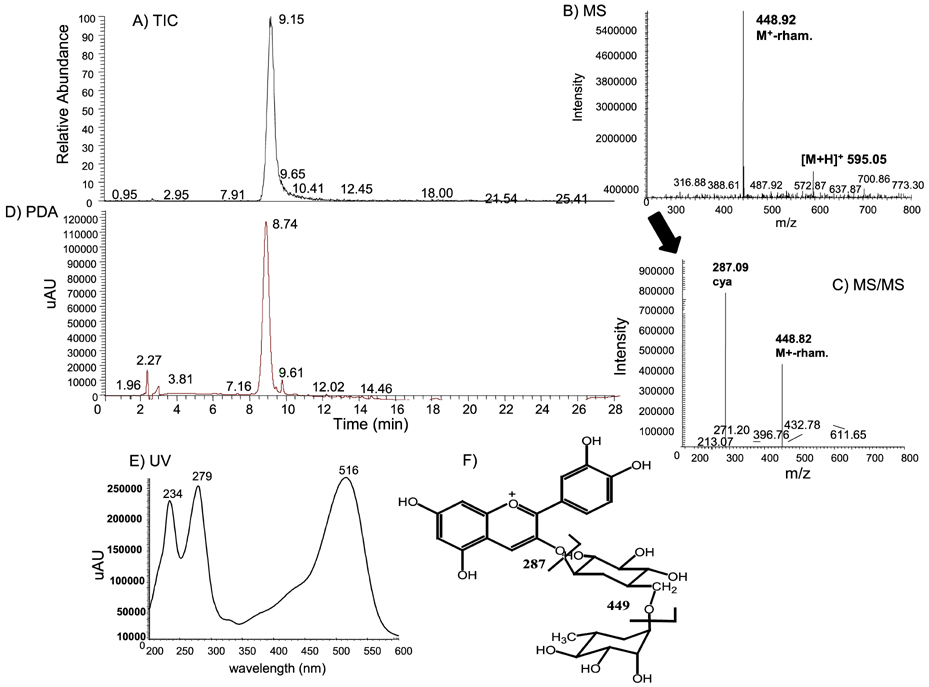
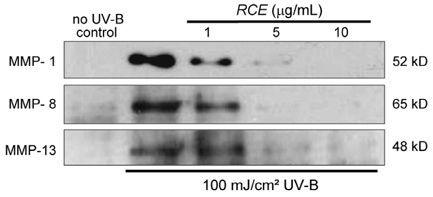
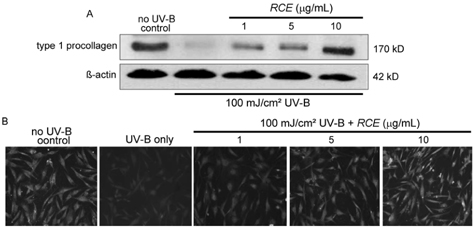
- Solar ultraviolet (UV) irradiation leads to distinct changes in the skin connective tissues by degradation of collagen, which is a major structural component in the extracellular matrix. UV irradiation induces the production of matrix metalloproteinases (MMP) capable of attacking native fibrillar collagen and responsible for inhibiting the construction of collagenous extracellular matrix. In this study, we attempted to investigate the protective actions of Rubus coreanus ethanol extract (RCE) on the MMP production and the consequent procollagen/collagen degradation in UV-B-irradiated human dermal fibroblasts. The analytical data showed that Rubus coreanus ethanol extract was mostly comprised of cyanidin 3-rutinoside. Pre-treatment of fibroblasts with this extract inhibited UV-B-induced production of MMP-1, MMP-8 and MMP-13 in dose-dependent manners. In addition, Western blot analysis and immunocytochemical staining assay revealed that RCE markedly augmented the cellular levels of procollagen/collagen declined in UV-B-exposed dermal fibroblasts. These results demonstrate that RCE blocks UV-B-induced increase of the collagen degradation by inhibiting MMP production. Thus, RCE may act as an agent inhibiting excessive dermal collagen degradation leading to the skin photoaging.
Keyword
MeSH Terms
Figure
Reference
-
1. Afaq F, Syed DN, Malik A, Hadi N, Sarfaraz S, Kweon MH, Khan N, Zaid MA, Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J Invest Dermatol. 2007. 127:222–232.
Article2. Anderson JJ, Garner SC. Phytoestrogens and bone. Baillieres Clin Endocrinol Metab. 1998. 12:543–557.3. Chau DY, Colliqhan RJ, Verderio EA, Addy VL, Griffin M. The cellular response to transglutaminase-cross-linked collagen. Biomaterials. 2005. 26:6518–6529.
Article4. Choi J, Lee KT, Ha J, Yun SY, Ko CD, Jung HJ, Park HJ. Antinociceptive and antiinflammatory effects of Niga-ichigoside F1 and 23-hydroxytormentic acid obtained from Rubus coreanus. Biol Pharm Bull. 2003. 26:1436–1441.
Article5. Choi YJ, Jeong YJ, Lee YJ, Kwon HM, Kang YH. (-)Epigallocatechin gallate and quercetin enhance survival signaling in response to oxidant-induced human endothelial apoptosis. J Nutr. 2005. 135:707–713.
Article6. Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce AP-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Investig Dermatol Symp Proc. 1998. 3:61–68.
Article7. Harman D. Free radical theory of aging. Mutat Res. 1992. 275:257–266.
Article8. Ho JN, Lee YH, Park JS, Jun WJ, Kim HK, Hong BS, Shin DH, Cho HY. Protective effects of aucubin isolated from Eucommia ulmoides against UVB-induced oxidative stress in human skin fibroblasts. Biol Pharm Bull. 2005. 28:1244–1248.
Article9. Hong V, Wrolstad RE. Use of HPLC separation/photodiode array detection for characterization of anthocyanins. J Agric Food Chem. 1990. 38:708–715.
Article10. Hsu S. Green tea and the skin. J Am Acad Dermatol. 2005. 52:1049–1059.
Article11. Huang C, Ma WY, Dawson MI, Rincon M, Flavell RA, Dong Z. Blocking activator protein-1 activity, but not activation retinoic acid response element, is required for the actitumor promotion effect of retinoic acid. Proc Natl Acad Sci. 1997. 94:5826–5830.
Article12. Huang C, Zhang D, Li J, Tong Q, Stoner GD. Differential inhibition of UV-induced activation of NF-κB and AP-1 by extracts from black raspberries, strawberries, and blueberries. Nutr Cancer. 2007. 58:205–212.
Article13. Jimenez F, Mitts TF, Liu K, Wang Y, Hinek A. Ellagic and tannic acids protect newly synthesized elastic fibers from premature enzymatic degradation in dermal fibroblast cultures. J Invest Dermatol. 2006. 126:1272–1280.
Article14. Lee SJ. Korean Folk Medicine. 1996. Seoul, Korea: Publishing Center of Seoul National University.15. Moon GS. Constituents and Uses of Medicinal Herbs. 1991. Seoul, Korea: Ilweolseogak;310–311.16. Nam JH, Jung HJ, Choi J, Lee KT, Park HJ. The antigastropathic and anti-rheumatic effect of niga-ichigoside F1 and 23-hydroxytormentic acid isolated from the unripe fruits of Rubus coreanus in a rat model. Biol Pharm Bull. 2006. 29:967–970.
Article17. Narayanan BA, Geoffroy O, Willingham MC, Re GG, Nixon DW. p53/p21 (WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999. 136:215–221.
Article18. Offord EA, Gautier JC, Avanti O, Scaletta C, Runge F, Kramer K, Applegate LA. Photo-protective potential of lycopene, β-carotene, vitamin E, vitamin C and carnosic acid in UV A-irradiated human skin fibroblasts. Free Radic Biol Med. 2002. 32:1293–1303.
Article19. Oh JH, Chung AS, Steinbrenner H, Sies H, Brenneisen P. Thioredoxin secreted upon ultraviolet A irradiation modulates activities of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in human dermal fibroblasts. Arch Biochem Biophys. 2004. 423:218–226.
Article20. Paivarinta E, Pajari AM, Torronen R, Mutanen M. Ellagic acid and natural sources of ellagitannins as possible chemopreventive agents against intestinal tumorigenesis in the Min mouse. Nutr Cancer. 2006. 54:79–83.
Article21. Park JH, Oh SM, Lim SS, Lee YS, Shin HK, Oh YS, Choe NH, Park JH, Kim JK. Induction of heme oxygenase-1 mediates the anti-inflammatory effects of the ethanol extract of Rubus coreanus in murine macrophages. Biochem Biophys Res Commun. 2006. 351:146–152.
Article22. Prockop DJ, Kivirikko KI, Tuderman L, Guzman NA. The biosynthesis of collagen and its disorders. N Engl J Med. 1979. 301:77–85.
Article23. Rogers GS, Gilchrest BA. The senile epidermis:environmental influences on skin aging and cutaneous carcinogenesis. Br J Dermatol. 1990. 122:55–60.
Article24. Saito Y, Shiga A, Yoshida Y, Furuhashi T, Fugita Y, Niki E. Effects of novel gaseous antioxidative system containing a Rosemary extract on the oxidation induced by nitrogen dioxide and ultraviolet radiation. Biosci Biotechnol Biochem. 2004. 68:781–786.
Article25. Skupien K, Oszmianski J, Kostrzewa-Nowak D, Tarasiuk J. In vitro antileukaemic activity of extracts from berry plantleaves against sensitive and multidrug resistant HL60 cells. Cancer Lett. 2006. 236:282–291.26. Torre LC, Barritt BH. Quantitative evaluation of rubus fruit. anthocyanin pigments. J Food Sci. 1977. 42:488–490.
Article27. Varani J, Perone P, Fligiel SE, Fisher GJ, Voorhees JJ. Inhibition of type 1 procollagen production in photodamage: correlation between presence of high molecular weight collagen fragments and reduced procollagen synthesis. J Invest Dermatol. 2002. 119:122–129.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory effect of Aralia elata ethanol extract against skin damage in UVB-exposed human keratinocytes and human dermal fibroblasts
- Behavior of Fibroblasts on a Porous Hyaluronic Acid Incorporated Collagen Matrix
- Growth inhibition effect of Rubus coreanus Miquel on Candida albicans
- Treatment for Two Cases of Acne Vulgaris with Rubus Coreanus Miquel Extract
- Physiological Functionalities of Vitis hybrid (Sheridan)-Rubus coreanus Red Wine Made by Saccharomyces cerevisiae