Cancer Res Treat.
2010 Sep;42(3):163-171.
Influence of Reduced Folate Carrier and Dihydrofolate Reductase Genes on Methotrexate-Induced Cytotoxicity
- Affiliations
-
- 1Laboratory of Medical Oncology, Catholic Research Institute of Medical Science, Seoul St. Mary's Hospital, The Catholic University of Korea School of Medicine, Seoul, Korea. jinkang@catholic.ac.kr
- 2Department of Medical Oncology, Seoul St. Mary's Hospital, The Catholic University of Korea School of Medicine, Seoul, Korea.
Abstract
- PURPOSE
The aim of this study is to investigate the effect of genetic variations and the expression of the reduced folate carrier (RFC) and dihydrofolate reductase (DHFR) on the drug sensitivity to methotrexate (MTX) in different cancer cell lines.
MATERIALS AND METHODS
We examined the six human cancer cell lines (MCF-7, AGS, A549, NCI-H23, HCT-116 and Saos-2). The cytotoxicity of MTX was measured by sulforhodamine B (SRB) assay. The expressions of the DHFR and RFC were evaluated by real-time PCR and western blotting. Four single nucleotide polymorphisms (SNPs) of the DHFR and two SNPs of the RFC were genotyped.
RESULTS
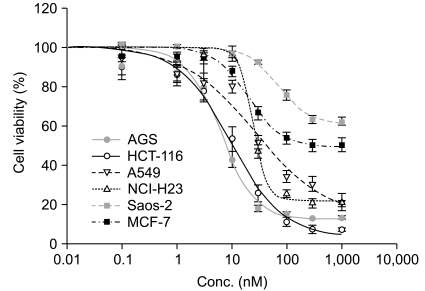
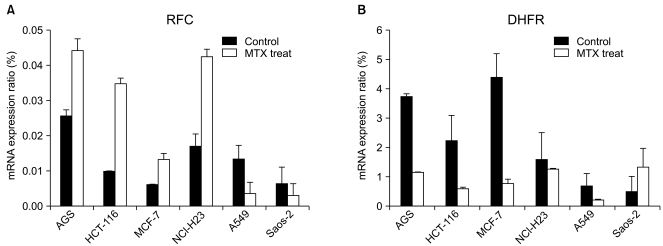
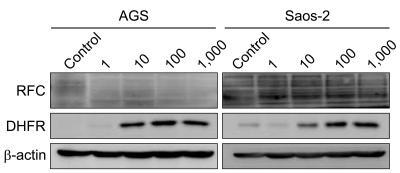
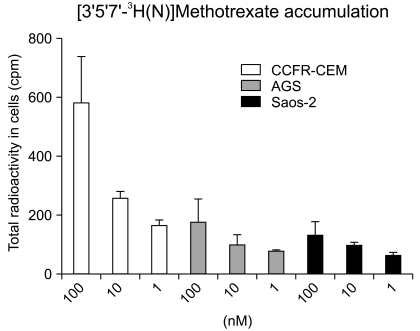
The IC50s of MTX was in an extensively broad range from 6.05+/-0.81 nM to>1,000 nM in the cell lines. The Saos-2 (>1,000 nM) and MCF-7 (114.31+/-5.34 nM) cells were most resistant to MTX; in contrast, the AGS and HCT-116 cells were highly sensitive to MTX with an IC50 of 6.05+/-0.81 nM and 13.56+/-3.76 nM, respectively. A reciprocal change of the RFC and DHFR mRNA expression was found between the MTX-sensitive AGS and MTX-resistant Saos-2 cells. There was no significant difference in the expression levels of RFC protein in both the AGS and Saos-2 cells, whereas DHFR protein was more increased in the MTX-resistant Saos-2 cells treated with MTX. The genotype of the MTX-sensitive AGS cells were mutant variants of the DHFR; in contrast, the Saos-2 cells had the wild-type of the DHFR.
CONCLUSION
In conclusion, this study showed that inverse change of the RFC and DHFR mRNA and protein expression was associated with RFC and DHFR polymorphisms and it is postulated that this phenomenon might play an important role in sensitivity of certain cancers to MTX.
Keyword
MeSH Terms
-
Blotting, Western
Cell Line
Folic Acid
Genetic Variation
Genotype
HCT116 Cells
Humans
Inhibitory Concentration 50
Methotrexate
Polymorphism, Single Nucleotide
Real-Time Polymerase Chain Reaction
Reduced Folate Carrier Protein
Rhodamines
RNA, Messenger
Tetrahydrofolate Dehydrogenase
Folic Acid
Methotrexate
RNA, Messenger
Reduced Folate Carrier Protein
Rhodamines
Tetrahydrofolate Dehydrogenase
Figure
Reference
-
1. Goto Y, Yue L, Yokoi A, Nishimura R, Uehara T, Koizumi S, et al. A novel single-nucleotide polymorphism in the 3'-untranslated region of the human dihydrofolate reductase gene with enhanced expression. Clin Cancer Res. 2001; 7:1952–1956. PMID: 11448909.2. Selga E, Noé V, Ciudad CJ. Transcriptional regulation of aldo-keto reductase 1C1 in HT29 human colon cancer cells resistant to methotrexate: role in the cell cycle and apoptosis. Biochem Pharmacol. 2008; 75:414–426. PMID: 17945194.
Article3. Serra M, Reverter-Branchat G, Maurici D, Benini S, Shen JN, Chano T, et al. Analysis of dihydrofolate reductase and reduced folate carrier gene status in relation to methotrexate resistance in osteosarcoma cells. Ann Oncol. 2004; 15:151–160. PMID: 14679136.
Article4. Padmanabhan S, Tripathi DN, Vikram A, Ramarao P, Jena GB. Methotrexate-induced cytotoxicity and genotoxicity in germ cells of mice: intervention of folic and folinic acid. Mutat Res. 2009; 673:43–52. PMID: 19110071.
Article5. Izbicka E, Diaz A, Streeper R, Wick M, Campos D, Steffen R, et al. Distinct mechanistic activity profile of pralatrexate in comparison to other antifolates in in vitro and in vivo models of human cancers. Cancer Chemother Pharmacol. 2009; 64:993–999. PMID: 19221750.
Article6. Affleck JG, Nowickyj SM, Walker VK. Selection for methotrexate resistance in mammalian cells bearing a Drosophila dihydrofolate reductase transgene: methotrexate resistance in transgenic mammalian cells. Cell Biol Toxicol. 2010; 26:117–126. PMID: 19337845.7. Yang R, Li WW, Hoang BH, Kim H, Banerjee D, Kheradpour A, et al. Quantitative correlation between promoter methylation and messenger RNA levels of the reduced folate carrier. BMC Cancer. 2008; 8:124. PMID: 18452618.
Article8. Scionti I, Michelacci F, Pasello M, Hattinger CM, Alberghini M, Manara MC, et al. Clinical impact of the methotrexate resistance-associated genes C-MYC and dihydrofolate reductase (DHFR) in high-grade osteosarcoma. Ann Oncol. 2008; 19:1500–1508. PMID: 18385200.
Article9. Dulucq S, St-Onge G, Gagné V, Ansari M, Sinnett D, Labuda D, et al. DNA variants in the dihydrofolate reductase gene and outcome in childhood ALL. Blood. 2008; 111:3692–3700. PMID: 18096764.
Article10. Ongaro A, De Mattei M, Della Porta MG, Rigolin G, Ambrosio C, Di Raimondo F, et al. Gene polymorphisms in folate metabolizing enzymes in adult acute lymphoblastic leukemia: effects on methotrexate-related toxicity and survival. Haematologica. 2009; 94:1391–1398. PMID: 19648163.
Article11. Jansen G, Westerhof GR, Jarmuszewski MJ, Kathmann I, Rijksen G, Schornagel JH. Methotrexate transport in variant human CCRF-CEM leukemia cells with elevated levels of the reduced folate carrier. Selective effect on carrier-mediated transport of physiological concentrations of reduced folates. J Biol Chem. 1990; 265:18272–18277. PMID: 2211701.
Article12. Backus HH, Pinedo HM, Wouters D, Padrón JM, Molders N, van Der Wilt CL, et al. Folate depletion increases sensitivity of solid tumor cell lines to 5-fluorouracil and antifolates. Int J Cancer. 2000; 87:771–778. PMID: 10956384.
Article13. Marshall LA, Rhee MS, Hofmann L, Khodjakov A, Schneider E. Increased lysosomal uptake of methotrexate-polyglutamates in two methotrexate-resistant cell lines with distinct mechanisms of resistance. Biochem Pharmacol. 2005; 71:203–213. PMID: 16263093.
Article14. Hattinger CM, Stoico G, Michelacci F, Pasello M, Scionti I, Remondini D, et al. Mechanisms of gene amplification and evidence of coamplification in drug-resistant human osteosarcoma cell lines. Genes Chromosomes Cancer. 2009; 48:289–309. PMID: 19105235.
Article15. Gomes CM, van Paassen H, Romeo S, Welling MM, Feitsma RI, Abrunhosa AJ, et al. Multidrug resistance mediated by ABC transporters in osteosarcoma cell lines: mRNA analysis and functional radiotracer studies. Nucl Med Biol. 2006; 33:831–840. PMID: 17045162.
Article16. Kang MH, Harutyunyan N, Hall CP, Papa RA, Lock RB. Methotrexate and aminopterin exhibit similar in vitro and in vivo preclinical activity against acute lymphoblastic leukaemia and lymphoma. Br J Haematol. 2009; 145:389–393. PMID: 19298590.17. Rothem L, Ifergan I, Kaufman Y, Priest DG, Jansen G, Assaraf YG. Resistance to multiple novel antifolates is mediated via defective drug transport resulting from clustered mutations in the reduced folate carrier gene in human leukaemia cell lines. Biochem J. 2002; 367:741–750. PMID: 12139489.
Article18. Patiño-García A, Zalacaín M, Marrodán L, San-Julián M, Sierrasesúmaga L. Methotrexate in pediatric osteosarcoma: response and toxicity in relation to genetic polymorphisms and dihydrofolate reductase and reduced folate carrier 1 expression. J Pediatr. 2009; 154:688–693. PMID: 19159907.
Article19. Lee C, Schwartz MP, Prakash S, Iwakura M, Matouschek A. ATP-dependent proteases degrade their substrates by processively unraveling them from the degradation signal. Mol Cell. 2001; 7:627–637. PMID: 11463387.
Article20. Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000; 19:94–102. PMID: 10619848.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of low dose folic acid replacement treatment on plasm homocysteine level in korean coronary artery disease patients.
- Intrathecal Chemotherapy Related Myelopathy Improved With Folate and Cyanocobalamin
- Fatal multiorgan dysfunction following repeated iodinated radiocontrast injection in a patient receiving low-dose oral methotrexate -a case report-
- Homocysteine, folate, and methylenetetrahydrofolate reductase polymorphism in korean normal subjects
- Enzymes involved in folate metabolism and its implication for cancer treatment