Allergy Asthma Immunol Res.
2015 Jan;7(1):14-21. 10.4168/aair.2015.7.1.14.
Chitotriosidase in the Pathogenesis of Inflammation, Interstitial Lung Diseases and COPD
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, New York-Presbyterian Hospital-Weill Cornell Medical Center, Weill Cornell Medical College, New York, NY, USA.
- 2Division of Pulmonary, Critical Care and Sleep Medicine, Department of Internal Medicine, New York University School of Medicine, New York, NY, USA.
- 3New York University, School of Medicine, Department of Environmental Medicine, Tuxedo Park, NY, USA.
- 4Bureau of Health Services and Office of Medical Affairs Fire Department of New York, Brooklyn, NY, USA.
- 5Molecular Microbiology and Immunology, Brown University,Warren Alpert School of Medicine Box G-L, Providence, RI, USA. chun_lee@brown.edu
- KMID: 2260147
- DOI: http://doi.org/10.4168/aair.2015.7.1.14
Abstract
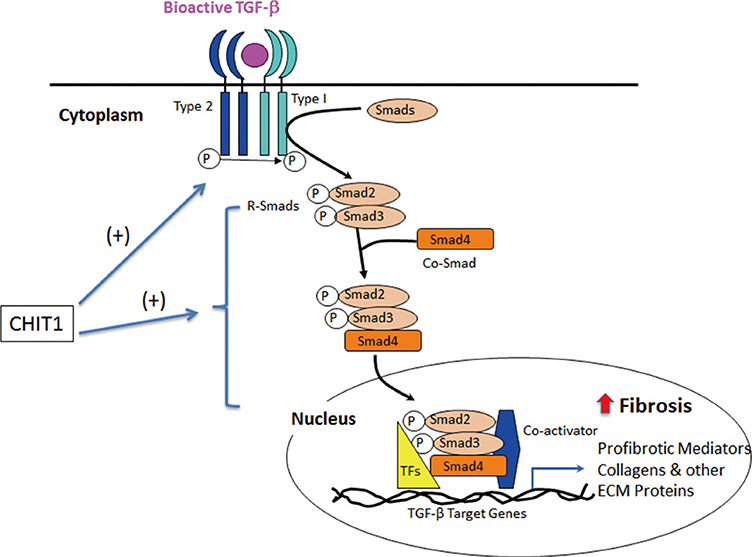
- As a member of 18 glycosyl hydrolase (GH) family, chitotriosidase (Chitinase 1, CHIT1) is a true chitinase mainly expressed in the differentiated and polarized macrophages. CHIT1 is an innate immune mediator that digests the cell walls of chitin-containing eukaryotic pathogens, such as fungi. However, CHIT1 is dysregulated in granulomatous and fibrotic interstitial lung diseases characterized by inflammation and tissue remodeling. These include tuberclosis, sarcoidosis, idiopathic pulmonary fibrosis, scleroderma-associated interstitial lung diseases (SSc-ILD), and chronic obstructive lung diseases (COPD). CHIT1 serum concentration correlates with the progression or the severity of these diseases, suggesting a potential use of CHIT1 as a biomarker or a therapeutic target. Recent studies with genetically modified mice demonstrate that CHIT1 enhances TGF-beta1 receptor expression and signaling, suggesting a role in initiating or amplifying the response to organ injury and repair. This additional CHIT1 activity is independent of its enzymatic activity. These studies suggest that CHIT1 serves a bridging function; it is both an innate immune mediator and a regulator of tissue remodeling. This review will focus on recent data linking CHIT1 to the pathogenesis of inflammation, interstitial lung disease, and COPD.
Keyword
MeSH Terms
-
Animals
Cell Wall
Chitinase
Fungi
Humans
Idiopathic Pulmonary Fibrosis
Inflammation*
Lung Diseases, Interstitial*
Lung Diseases, Obstructive
Macrophages
Mice
Pulmonary Disease, Chronic Obstructive*
Sarcoidosis
Transforming Growth Factor beta
Transforming Growth Factor beta1
Chitinase
Transforming Growth Factor beta
Transforming Growth Factor beta1
Figure
Reference
-
1. Boot RG, Bussink AP, Verhoek M, de Boer PA, Moorman AF, Aerts JM. Marked differences in tissue-specific expression of chitinases in mouse and man. J Histochem Cytochem. 2005; 53:1283–1292.2. Fusetti F, von Moeller H, Houston D, Rozeboom HJ, Dijkstra BW, Boot RG, et al. Structure of human chitotriosidase. Implications for specific inhibitor design and function of mammalian chitinase-like lectins. J Biol Chem. 2002; 277:25537–25544.3. Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011; 73:479–501.4. Bussink AP, Speijer D, Aerts JM, Boot RG. Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics. 2007; 177:959–970.5. Funkhouser JD, Aronson NN Jr. Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol. 2007; 7:96.6. Di Rosa M, Musumeci M, Scuto A, Musumeci S, Malaguarnera L. Effect of interferon-gamma, interleukin-10, lipopolysaccharide and tumor necrosis factor-alpha on chitotriosidase synthesis in human macrophages. Clin Chem Lab Med. 2005; 43:499–502.7. Malaguarnera L, Musumeci M, Di Rosa M, Scuto A, Musumeci S. Interferon-gamma, tumor necrosis factor-alpha, and lipopolysaccharide promote chitotriosidase gene expression in human macrophages. J Clin Lab Anal. 2005; 19:128–132.8. Renkema GH, Boot RG, Muijsers AO, Donker-Koopman WE, Aerts JM. Purification and characterization of human chitotriosidase, a novel member of the chitinase family of proteins. J Biol Chem. 1995; 270:2198–2202.9. van Eijk M, van Roomen CP, Renkema GH, Bussink AP, Andrews L, Blommaart EF, et al. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int Immunol. 2005; 17:1505–1512.10. Kanneganti M, Kamba A, Mizoguchi E. Role of chitotriosidase (chitinase 1) under normal and disease conditions. Epithel Biol Pharmacol. 2012; 5:1–9.11. Lee CG, Herzog EL, Ahangari F, Zhou Y, Gulati M, Lee CM, et al. Chitinase 1 is a biomarker for and therapeutic target in scleroderma-associated interstitial lung disease that augments TGF-beta1 signaling. J Immunol. 2012; 189:2635–2644.12. van Eijk M, Scheij SS, van Roomen CP, Speijer D, Boot RG, Aerts JM. TLR- and NOD2-dependent regulation of human phagocyte-specific chitotriosidase. FEBS letters. 2007; 581:5389–5395.13. Artieda M, Cenarro A, Gañán A, Jericó I, Gonzalvo C, Casado JM, et al. Serum chitotriosidase activity is increased in subjects with atherosclerosis disease. Arterioscler Thromb Vasc Biol. 2003; 23:1645–1652.14. Canudas J, Cenarro A, Civeira F, Garcí-Otín AL, Arístegui R, Díaz C, et al. Chitotriosidase genotype and serum activity in subjects with combined hyperlipidemia: effect of the lipid-lowering agents, atorvastatin and bezafibrate. Metabolism. 2001; 50:447–450.15. Lee P, Waalen J, Crain K, Smargon A, Beutler E. Human chitotriosidase polymorphisms G354R and A442V associated with reduced enzyme activity. Blood Cells Mol Dis. 2007; 39:353–360.16. Malaguarnera L, Simporè J, Prodi DA, Angius A, Sassu A, Persico I, et al. A 24-bp duplication in exon 10 of human chitotriosidase gene from the sub-Saharan to the Mediterranean area: role of parasitic diseases and environmental conditions. Genes Immun. 2003; 4:570–574.17. Boot RG, Renkema GH, Verhoek M, Strijland A, Bliek J, de Meulemeester TM, et al. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J Biol Chem. 1998; 273:25680–25685.18. Choi EH, Zimmerman PA, Foster CB, Zhu S, Kumaraswami V, Nutman TB, et al. Genetic polymorphisms in molecules of innate immunity and susceptibility to infection with Wuchereria bancrofti in South India. Genes Immun. 2001; 2:248–253.19. Labadaridis I, Dimitriou E, Theodorakis M, Kafalidis G, Velegraki A, Michelakakis H. Chitotriosidase in neonates with fungal and bacterial infections. Arch Dis Child Fetal Neonatal Ed. 2005; 90:F531–F532.20. Ober C, Chupp GL. The chitinase and chitinase-like proteins: a review of genetic and functional studies in asthma and immune-mediated diseases. Curr Opin Allergy Clin Immunol. 2009; 9:401–408.21. Lehrnbecher T, Bernig T, Hanisch M, Koehl U, Behl M, Reinhardt D, et al. Common genetic variants in the interleukin-6 and chitotriosidase genes are associated with the risk for serious infection in children undergoing therapy for acute myeloid leukemia. Leukemia. 2005; 19:1745–1750.22. Kim KW, Park J, Lee JH, Lee HS, Lee J, Lee KH, et al. Association of genetic variation in chitotriosidase with atopy in Korean children. Ann Allergy Asthma Immunol. 2013; 110:444–449.e1.23. Hollak CE, van Weely S, van Oers MH, Aerts JM. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest. 1994; 93:1288–1292.24. Hollak CE, Maas M, Aerts JM. Clinically relevant therapeutic endpoints in type I Gaucher disease. J Inherit Metab Dis. 2001; 24:Suppl 2. 97–105.25. de Fost M, Hollak CE, Groener JE, Aerts JM, Maas M, Poll LW, et al. Superior effects of high-dose enzyme replacement therapy in type 1 Gaucher disease on bone marrow involvement and chitotriosidase levels: a 2-center retrospective analysis. Blood. 2006; 108:830–835.26. Pastores GM, Barnett NL. Current and emerging therapies for the lysosomal storage disorders. Expert Opin Emerg Drugs. 2005; 10:891–902.27. Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights. 2007; 2:128–146.28. Agapov E, Battaile JT, Tidwell R, Hachem R, Patterson GA, Pierce RA, et al. Macrophage chitinase 1 stratifies chronic obstructive lung disease. Am J Respir Cell Mol Biol. 2009; 41:379–384.29. Létuvé S, Kozhich A, Humbles A, Brewah Y, Dombret MC, Grandsaigne M, et al. Lung chitinolytic activity and chitotriosidase are elevated in chronic obstructive pulmonary disease and contribute to lung inflammation. Am J Pathol. 2010; 176:638–649.30. Cho SJ, Nolan A, Echevarria GC, Kwon S, Naveed B, Schenck E, et al. Chitotriosidase is a biomarker for the resistance to World Trade Center lung injury in New York City firefighters. J Clin Immunol. 2013; 33:1134–1142.31. Lee CM, Park JW, Cho WK, Zhou Y, Han B, Yoon PO, et al. Modifiers of TGF-beta1 effector function as novel therapeutic targets of pulmonary fibrosis. Korean J Intern Med. 2014; 29:281–290.32. Salfinger M, Hale YM, Driscoll JR. Diagnostic tools in tuberculosis. Present and future. Respiration. 1998; 65:163–170.33. Rivas-Santiago B, Vieyra-Reyes P, Araujo Z. Cell immunity response in human pulmonary tuberculosis. Review. Invest Clin. 2005; 46:391–412.34. Rajavelu P, Das SD. Th2-type immune response observed in healthy individuals to sonicate antigen prepared from the most prevalent Mycobacterium tuberculosis strain with single copy of IS6110. FEMS Immunol Med Microbiol. 2005; 45:95–102.35. Cakir G, Gumus S, Ucar E, Kaya H, Tozkoparan E, Akgul EO, et al. Serum chitotriosidase activity in pulmonary tuberculosis: response to treatment and correlations with clinical parameters. Ann Lab Med. 2012; 32:184–189.36. Tasci C, Tapan S, Ozkaya S, Demirer E, Deniz O, Balkan A, et al. Efficacy of serum chitotriosidase activity in early treatment of patients with active tuberculosis and a negative sputum smear. Ther Clin Risk Manag. 2012; 8:369–372.37. Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, et al. American Thoracic Society. European Respiratory Society. World Association of Sarcoidosis and other Granulomatous Disorders. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999; 16:149–173.38. Lynch JP 3rd, Kazerooni EA, Gay SE. Pulmonary sarcoidosis. Clin Chest Med. 1997; 18:755–785.39. Judson MA, Baughman RP, Thompson BW, Teirstein AS, Terrin ML, Rossman MD, et al. Two year prognosis of sarcoidosis: the ACCESS experience. Sarcoidosis Vasc Diffuse Lung Dis. 2003; 20:204–211.40. Corte TJ, Wells AU, Nicholson AG, Hansell DM, Wort SJ. Pulmonary hypertension in sarcoidosis: a review. Respirology. 2011; 16:69–77.41. Agostini C, Semenzato G. Cytokines in sarcoidosis. Semin Respir Infect. 1998; 13:184–196.42. Ziegenhagen MW, Rothe ME, Schlaak M, Müller-Quernheim J. Bronchoalveolar and serological parameters reflecting the severity of sarcoidosis. Eur Respir J. 2003; 21:407–413.43. Rottoli P, Magi B, Perari MG, Liberatori S, Nikiforakis N, Bargagli E, et al. Cytokine profile and proteome analysis in bronchoalveolar lavage of patients with sarcoidosis, pulmonary fibrosis associated with systemic sclerosis and idiopathic pulmonary fibrosis. Proteomics. 2005; 5:1423–1430.44. Kantrow SP, Meyer KC, Kidd P, Raghu G. The CD4/CD8 ratio in BAL fluid is highly variable in sarcoidosis. Eur Respir J. 1997; 10:2716–2721.45. Agostini C, Trentin L, Facco M, Sancetta R, Cerutti A, Tassinari C, et al. Role of IL-15, IL-2, and their receptors in the development of T cell alveolitis in pulmonary sarcoidosis. J Immunol. 1996; 157:910–918.46. Straub JP, van Kamp GJ, van Maarsseveen TC, Stam J. Biochemical parameters in BAL of sarcoisosis. Sarcoidosis. 1995; 12:51–57.47. Bargagli E, Maggiorelli C, Rottoli P. Human chitotriosidase: a potential new marker of sarcoidosis severity. Respiration. 2008; 76:234–238.48. Dai H, Guzman J, Chen B, Costabel U. Production of soluble tumor necrosis factor receptors and tumor necrosis factor-alpha by alveolar macrophages in sarcoidosis and extrinsic allergic alveolitis. Chest. 2005; 127:251–256.49. Ziegenhagen MW, Rothe ME, Zissel G, Müller-Quernheim J. Exaggerated TNFalpha release of alveolar macrophages in corticosteroid resistant sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002; 19:185–190.50. Grosso S, Margollicci MA, Bargagli E, Buccoliero QR, Perrone A, Galimberti D, et al. Serum levels of chitotriosidase as a marker of disease activity and clinical stage in sarcoidosis. Scand J Clin Lab Invest. 2004; 64:57–62.51. Bargagli E, Margollicci M, Perrone A, Luddi A, Perari MG, Bianchi N, et al. Chitotriosidase analysis in bronchoalveolar lavage of patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2007; 24:59–64.52. Bargagli E, Bennett D, Maggiorelli C, Di Sipio P, Margollicci M, Bianchi N, et al. Human chitotriosidase: a sensitive biomarker of sarcoidosis. J Clin Immunol. 2013; 33:264–270.53. Zissel G, Prasse A, Müller-Quernheim J. Sarcoidosis--immunopathogenetic concepts. Semin Respir Crit Care Med. 2007; 28:3–14.54. Lem VM, Lipscomb MF, Weissler JC, Nunez G, Ball EJ, Stastny P, et al. Bronchoalveolar cells from sarcoid patients demonstrate enhanced antigen presentation. J Immunol. 1985; 135:1766–1771.55. Ina Y, Takada K, Yamamoto M, Morishita M, Miyachi A. Antigen-presenting capacity in patients with sarcoidosis. Chest. 1990; 98:911–916.56. Venet A, Hance AJ, Saltini C, Robinson BW, Crystal RG. Enhanced alveolar macrophage-mediated antigen-induced T-lymphocyte proliferation in sarcoidosis. J Clin Invest. 1985; 75:293–301.57. Tchernev G, Ananiev J, Cardoso JC, Wollina U, Verma SB, Patterson JW, et al. Sarcoidosis and molecular mimicry--important etiopathogenetic aspects: current state and future directions. Wien Klin Wochenschr. 2012; 124:227–238.58. Ali MM, Atwan AA, Gonzalez ML. Cutaneous sarcoidosis: updates in the pathogenesis. J Eur Acad Dermatol Venereol. 2010; 24:747–755.59. Bargagli E, Bianchi N, Margollicci M, Olivieri C, Luddi A, Coviello G, et al. Chitotriosidase and soluble IL-2 receptor: comparison of two markers of sarcoidosis severity. Scand J Clin Lab Invest. 2008; 68:479–483.60. Strausz J, Müller-Quernheim J, Ferlinz R. Secreted interleukin-2 receptor as a parameter of the activity of sarcoidosis. Dtsch Med Wochenschr. 1989; 114:744–749.61. Boot RG, Hollak CE, Verhoek M, Alberts C, Jonkers RE, Aerts JM. Plasma chitotriosidase and CCL18 as surrogate markers for granulomatous macrophages in sarcoidosis. Clin Chim Acta. 2010; 411:31–36.62. Schutyser E, Richmond A, Van Damme J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J Leukoc Biol. 2005; 78:14–26.63. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment International consensus statement American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000; 161:646–664.64. American Thoracic Society. European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002; 165:277–304.65. Selman M, King TE, Pardo A. American Thoracic Society. European Respiratory Society. American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001; 134:136–151.66. Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, Goldmann T, et al. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006; 173:781–792.67. Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007; 66:940–944.68. Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000; 343:269–280.69. Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004; 364:709–721.70. Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004; 56:515–548.71. Seibold MA, Donnelly S, Solon M, Innes A, Woodruff PG, Boot RG, et al. Chitotriosidase is the primary active chitinase in the human lung and is modulated by genotype and smoking habit. The Journal of allergy and clinical immunology. 2008; 122:944–950.e3.72. Lee CG, Elias JA. Role of breast regression protein-39/YKL-40 in asthma and allergic responses. Allergy Asthma Immunol Res. 2010; 2:20–27.73. Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004; 304:1678–1682.74. Zimmermann N, Mishra A, King NE, Fulkerson PC, Doepker MP, Nikolaidis NM, et al. Transcript signatures in experimental asthma: identification of STAT6-dependent and -independent pathways. J Immunol. 2004; 172:1815–1824.75. Zhao J, Zhu H, Wong CH, Leung KY, Wong WS. Increased lungkine and chitinase levels in allergic airway inflammation: a proteomics approach. Proteomics. 2005; 5:2799–2807.76. Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008; 358:1682–1691.77. Shuhui L, Mok YK, Wong WS. Role of mammalian chitinases in asthma. Int Arch Allergy Immunol. 2009; 149:369–377.78. Livnat G, Bar-Yoseph R, Mory A, Dagan E, Elias N, Gershoni R, et al. Duplication in CHIT1 gene and the risk for Aspergillus lung disease in CF patients. Pediatr Pulmonol. 2014; 49:21–27.79. Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009; 206:1149–1166.80. Lee CG. Chitin, chitinases and chitinase-like proteins in allergic inflammation and tissue remodeling. Yonsei Med J. 2009; 50:22–30.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Year in Review of Interstitial Lung Diseases: Focused on Idiopathic Interstitial Pneumonia
- Pathogenesis and pathophysiology of COPD
- Role of Th17 Cell and Autoimmunity in Chronic Obstructive Pulmonary Disease
- A Case of Respiratory Bronchiolitis-Associated Interstitial Lung Disease
- COPD as a Disease of Immunosenescence