Korean J Hematol.
2012 Sep;47(3):186-193. 10.5045/kjh.2012.47.3.186.
Identification of genes underlying different methylation profiles in refractory anemia with excess blast and refractory cytopenia with multilineage dysplasia in myelodysplastic syndrome
- Affiliations
-
- 1Department of Internal Medicine, Dong-A University Medical Center, Busan, Korea. kimhj@dau.ac.kr
- 2Department of Laboratory Medicine, Dong-A University Medical Center, Busan, Korea.
- 3Cancer Genomics Branch, National Cancer Center, Goyang, Korea.
- 4Laboratory of Experimental Carcinogenesis, Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA.
- KMID: 2251952
- DOI: http://doi.org/10.5045/kjh.2012.47.3.186
Abstract
- BACKGROUND
Myelodysplastic syndrome (MDS) is a preleukemic condition that transforms into acute myeloid leukemia. However, the genetic events underlying this transformation remain poorly understood. Aberrant DNA methylation may play a causative role in the disease and its prognosis. Thus, we compared the DNA methylation profiles in refractory anemia with excess blast (RAEB) to those in refractory cytopenia with multilineage dysplasia (RCMD).
METHODS
Bone marrow samples were collected from 20 patients with primary MDS (9 with RAEB and 11 with RCMD), and peripheral blood samples were collected from 4 healthy controls. These samples were assessed using a commercial whole genome-wide methylation assay. Methylation-specific polymerase chain reaction (PCR) was used to detect the methylation of candidate gene promoters in RAEB and RCMD.
RESULTS
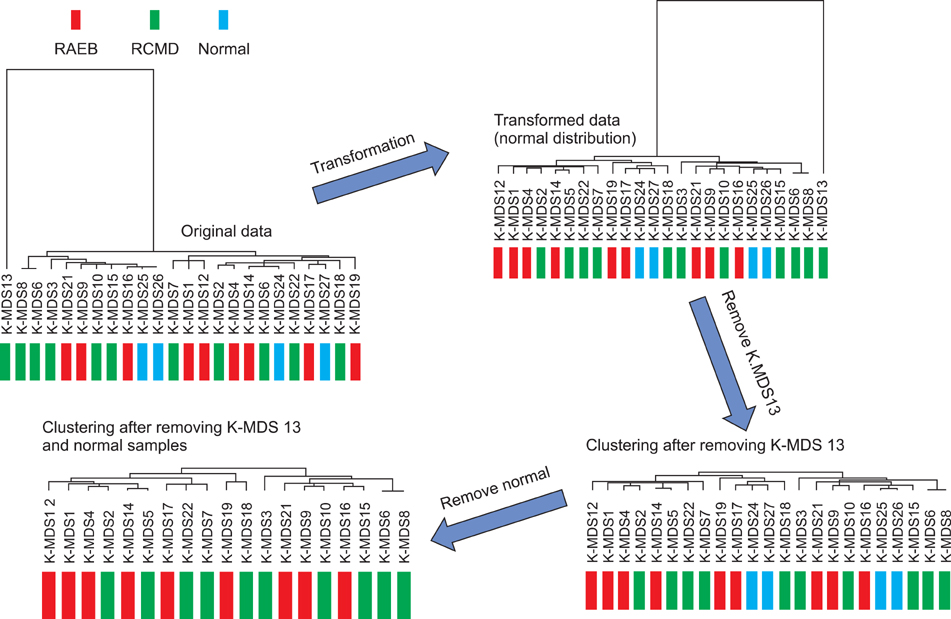
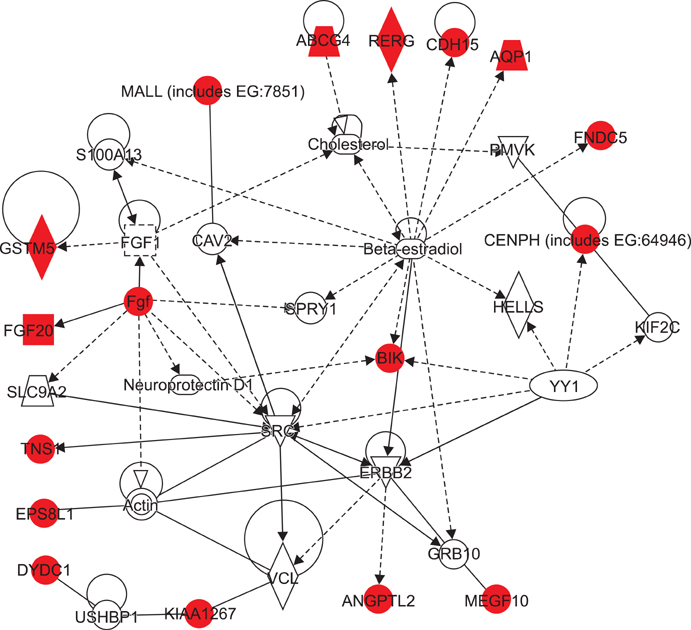
Microarray data revealed significant hypermethylation in 69 genes within RAEB but not RCMD. Candidate genes were mapped to 5 different networks, and network 1 had the highest score due to its involvement in gene expression, cancer, and cell cycle. Five genes (GSTM5, BIK, CENPH, RERG, and ANGPTL2) were associated with malignant disease progression. Among them, the methylated promoter pairs of GSTM5 (55.5% and 20%), BIK (20% and 0%), and ANGPTL2 (44.4% and 10%) were observed more frequently in RAEB.
CONCLUSION
DNA methylation of GSTM5, BIK, and ANGPTL2 may induce epigenetic silencing and contribute to the increasing blasts and resulting MDS progression; however, the functions of these genes were not determined. Further study focusing on epigenetic silencing using various detection modalities is required.
Keyword
MeSH Terms
Figure
Reference
-
1. Heaney ML, Golde DW. Myelodysplasia. N Engl J Med. 1999. 340:1649–1660.
Article2. Hedegaard J, Arce C, Bicciato S, et al. Methods for interpreting lists of affected genes obtained in a DNA microarray experiment. BMC Proc. 2009. 3:Suppl 4. S5.
Article3. Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996. 93:9821–9826.
Article4. Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005. 6:597–610.
Article5. Rush LJ, Plass C. Alterations of DNA methylation in hematologic malignancies. Cancer Lett. 2002. 185:1–12.
Article6. Nakamaki T, Bartram C, Seriu T, et al. Molecular analysis of the cyclin-dependent kinase inhibitor genes, p15, p16, p18 and p19 in the myelodysplastic syndromes. Leuk Res. 1997. 21:235–240.
Article7. Uchida T, Kinoshita T, Nagai H, et al. Hypermethylation of the p15INK4B gene in myelodysplastic syndromes. Blood. 1997. 90:1403–1409.
Article8. Quesnel B, Guillerm G, Vereecque R, et al. Methylation of the p15(INK4b) gene in myelodysplastic syndromes is frequent and acquired during disease progression. Blood. 1998. 91:2985–2990.
Article9. Aggerholm A, Holm MS, Guldberg P, Olesen LH, Hokland P. Promoter hypermethylation of p15INK4B, HIC1, CDH1, and ER is frequent in myelodysplastic syndrome and predicts poor prognosis in early-stage patients. Eur J Haematol. 2006. 76:23–32.
Article10. Jiang Y, Dunbar A, Gondek LP, et al. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood. 2009. 113:1315–1325.
Article11. Pellagatti A, Esoof N, Watkins F, et al. Gene expression profiling in the myelodysplastic syndromes using cDNA microarray technology. Br J Haematol. 2004. 125:576–583.
Article12. Schumacher A, Kapranov P, Kaminsky Z, et al. Microarray-based DNA methylation profiling: technology and applications. Nucleic Acids Res. 2006. 34:528–542.
Article13. Parl FF. Glutathione S-transferase genotypes and cancer risk. Cancer Lett. 2005. 221:123–129.
Article14. Den Boer ML, Pieters R, Kazemier KM, et al. Different expression of glutathione S-transferase alpha, mu and pi in childhood acute lymphoblastic and myeloid leukaemia. Br J Haematol. 1999. 104:321–327.
Article15. Kearns PR, Chrzanowska-Lightowlers ZM, Pieters R, Veerman A, Hall AG. Mu class glutathione S-transferase mRNA isoform expression in acute lymphoblastic leukaemia. Br J Haematol. 2003. 120:80–88.
Article16. Peng DF, Razvi M, Chen H, et al. DNA hypermethylation regulates the expression of members of the Mu-class glutathione S-transferases and glutathione peroxidases in Barrett's adenocarcinoma. Gut. 2009. 58:5–15.
Article17. Kikuchi R, Tsuda H, Kozaki K, et al. Frequent inactivation of a putative tumor suppressor, angiopoietin-like protein 2, in ovarian cancer. Cancer Res. 2008. 68:5067–5075.
Article18. Zhang CC, Kaba M, Ge G, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006. 12:240–245.
Article19. Naumann U, Schmidt F, Wick W, et al. Adenoviral natural born killer gene therapy for malignant glioma. Hum Gene Ther. 2003. 14:1235–1246.
Article20. Gillissen B, Essmann F, Graupner V, et al. Induction of cell death by the BH3-only Bcl-2 homolog Nbk/Bik is mediated by an entirely Bax-dependent mitochondrial pathway. EMBO J. 2003. 22:3580–3590.
Article21. Tong Y, Yang Q, Vater C, et al. The pro-apoptotic protein, Bik, exhibits potent antitumor activity that is dependent on its BH3 domain. Mol Cancer Ther. 2001. 1:95–102.22. Kim TY, Zhong S, Fields CR, Kim JH, Robertson KD. Epigenomic profiling reveals novel and frequent targets of aberrant DNA methylation-mediated silencing in malignant glioma. Cancer Res. 2006. 66:7490–7501.
Article23. Dai Z, Liu S, Marcucci G, Sadee W. 5-Aza-2'-deoxycytidine and depsipeptide synergistically induce expression of BIK (BCL2-interacting killer). Biochem Biophys Res Commun. 2006. 351:455–461.
Article24. Lepelley P, Campergue L, Grardel N, Preudhomme C, Cosson A, Fenaux P. Is apoptosis a massive process in myelodysplastic syndromes? Br J Haematol. 1996. 95:368–371.
Article25. Rajapaksa R, Ginzton N, Rott LS, Greenberg PL. Altered oncoprotein expression and apoptosis in myelodysplastic syndrome marrow cells. Blood. 1996. 88:4275–4287.
Article26. Tsoplou P, Kouraklis-Symeonidis A, Thanopoulou E, Zikos P, Orphanos V, Zoumbos NC. Apoptosis in patients with myelodysplastic syndromes: differential involvement of marrow cells in 'good' versus 'poor' prognosis patients and correlation with apoptosis-related genes. Leukemia. 1999. 13:1554–1563.
Article27. Finlin BS, Gau CL, Murphy GA, et al. RERG is a novel ras-related, estrogen-regulated and growth-inhibitory gene in breast cancer. J Biol Chem. 2001. 276:42259–42267.
Article28. Wang AG, Fang W, Han YH, et al. Expression of the RERG gene is gender-dependent in hepatocellular carcinoma and regulated by histone deacetyltransferases. J Korean Med Sci. 2006. 21:891–896.
Article29. Benetatos L, Dasoula A, Syed N, Hatzimichael E, Crook T, Bourantas KL. Methylation analysis of the von Hippel-Lindau gene in acute myeloid leukaemia and myelodysplastic syndromes. Leukemia. 2008. 22:1293–1295.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Membranous glomerulonephritis in a patient with myelodysplastic syndrome-refractory cytopenia with multilineage dysplasia
- Bullous Pemphigoid in an Elderly Patient with Myelodysplastic Syndrome and Refractory Anemia Coupled with Excess of Blast
- G-Score and the Percentage of Pseudo-Pelgeroid Polymorphoneutrophils in Myelodysplastic Syndrome
- A Case of Myelodysplastic Syndrome Characterized by Hemolytic Anemia at Presentation
- Myelodysplastic Syndrome and Minimal Change Nephrotic Syndrome treated with Steroid Challenge