J Korean Ophthalmol Soc.
2010 Feb;51(2):276-281. 10.3341/jkos.2010.51.2.276.
Effect of Glucose on the Production of Reactive Oxygen Species in Retinal Pigment Epithelial Cells
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Catholic University of Daegu, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2213152
- DOI: http://doi.org/10.3341/jkos.2010.51.2.276
Abstract
- PURPOSE
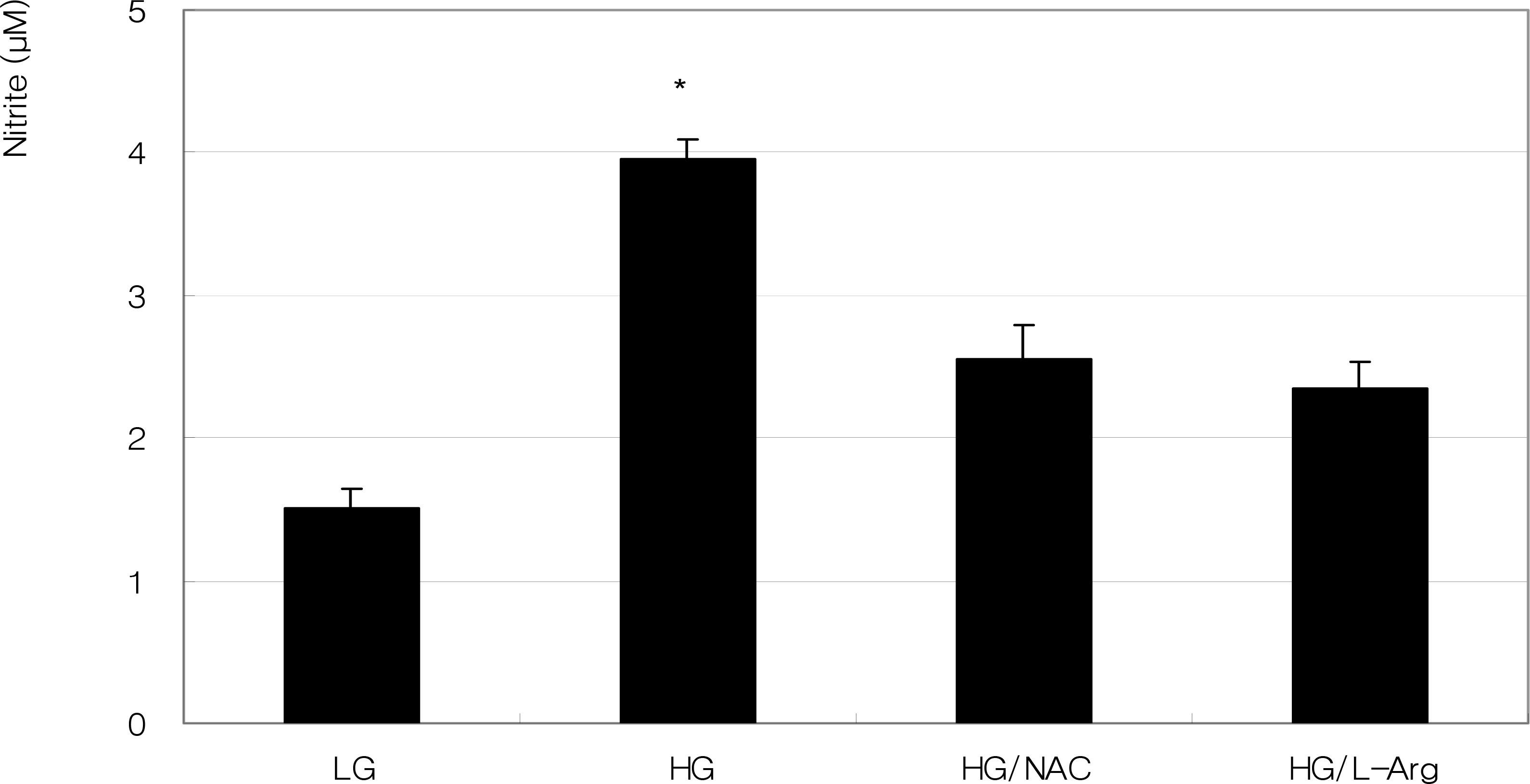
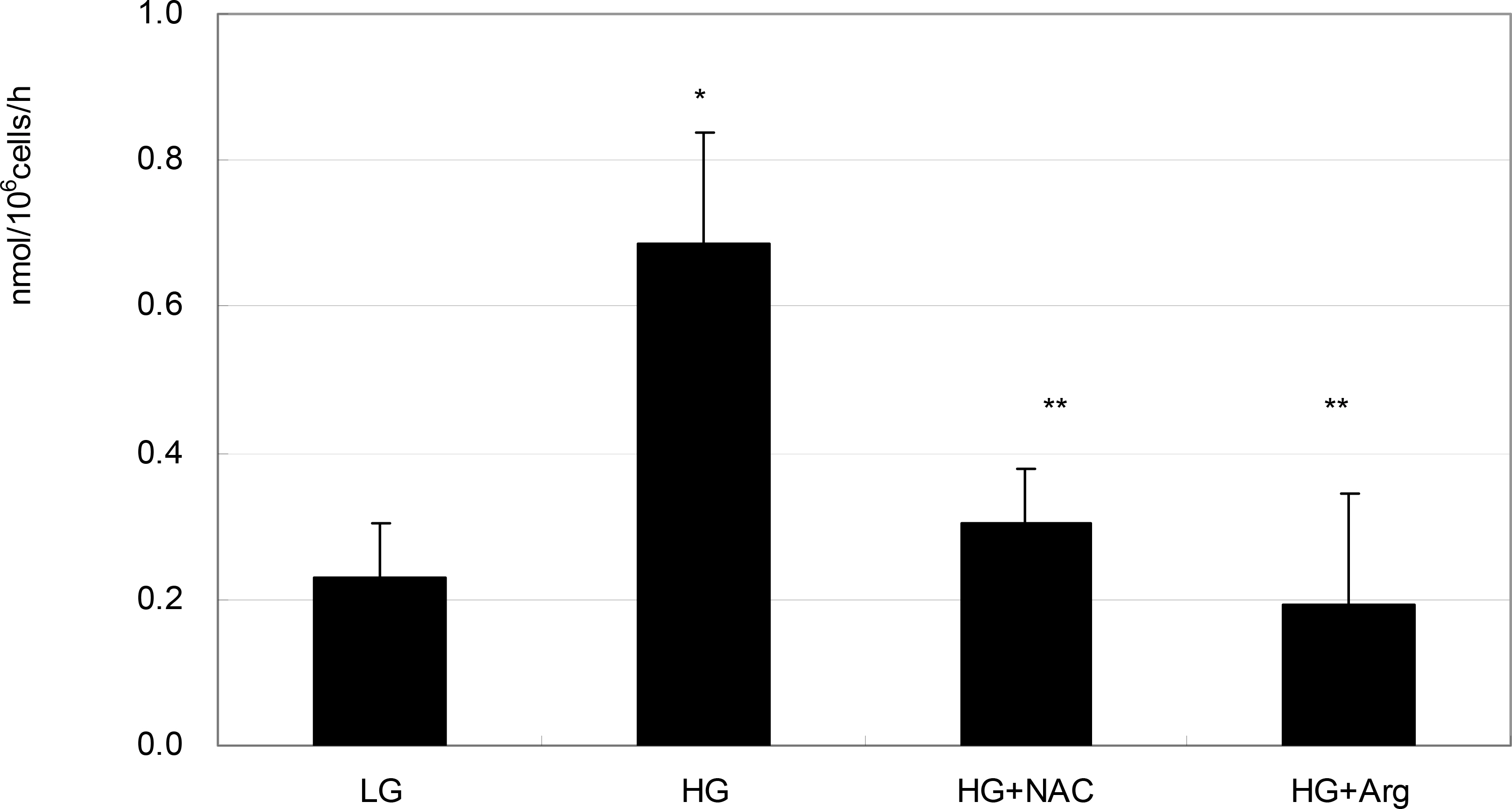
To investigate the effect of high glucose (HG) on the production of reactive oxygen species (ROS) in retinal pigment epithelial (RPE) cells.
METHODS
ARPE-19 cells were exposed to low glucose (5 mM) and high glucose (HG, 25 mM) for three days. Additionally, 50 micrometer N-acetyl cysteine (NAC) and 100 micrometer L-arginine were co-exposed. Productions of nitric oxide (NO), ROS, and superoxide were determined by Griess assay, DCFH-DA assay, and modified cytochrome c assay, respectively.
RESULTS
HG increased production of NO, ROS, and superoxide. HG-induced cells increased production of superoxide and ROS but were suppressed by NAC and L-arginine (substrate for NO production) as an antioxidant.
CONCLUSIONS
HG increased ROS production in RPE cells. Thus, HG may cause cellular dysfunction and damage by inducing oxidative stress in RPE cells.
MeSH Terms
Figure
Cited by 1 articles
-
Cytoprotective Effect of Polyphenolic Compounds against Oxidative Stress in Cultured Retinal Pigment Epithelial Cells
Kyung Hoon Seo, Seung Young Yu, Hyung Woo Kwak
J Korean Ophthalmol Soc. 2016;57(1):106-112. doi: 10.3341/jkos.2016.57.1.106.
Reference
-
References
1. Wei YH. Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med. 1998; 217:53–63.
Article2. Sacca SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007; 84:389–99.3. Sacca SC, Pascotto A, Camicione P, et al. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005; 123:458–63.4. Kawasaki A, Otori Y, Barnstable CJ. Müller cell protection of rat retinal ganglion cells from glutamate and nitric oxide aberrations. Invest Ophthalmol Vis Sci. 2000; 41:3444–50.5. Morgan J, Caprioli J, Koseki Y. Nitric oxide mediates excitotoxic and anoxic damage in rat retinal ganglion cells cocultured with astroglia. Arch Ophthalmol. 1999; 117:1524–9.
Article6. Vorwerk CK, Hyman BT, Miller JW, et al. The role of neuronal and endothelial nitric oxide synthase in retinal excitotoxicity. Invest Ophthalmol Vis Sci. 1997; 38:2038–44.7. Park GS, Kwon NS, Kim YM, Kim JC. The role of nitric oxide in ocular surface diseases. Korean J Ophthalmol. 2001; 15:59–66.
Article8. Becquet F, Courtois Y, Goureau O. Nitric oxide in the eye: Multifaceted roles and diverse outcomes. Surv Ophthalmol. 1997; 42:71–82.
Article9. Brodsky SV, Morrishow AM, Dharia N, et al. Glucose scavenging of nitric oxide. Am J Physiol Renal Physiol. 2001; 280:480–6.
Article10. El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci. 2003; 44:3135–43.
Article11. Becker B. Diabetes and primary open-angle glaucoma. Am J Ophthalmol. 1971; 1:1–16.12. Davies PD, Duncan G, Pynsent PB, et al. Aqueous humor glucose concentration in cataract patients and its effect on the lens. Exp Eye Res. 1984; 39:605–9.13. Schraermeyer U, Heiman K. Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment Cell Res. 1999; 12:219–36.
Article14. Proctor PH, Reynolds ES. Free radicals and disease in man. Physiol Chem Phy Med NMR. 1984; 16:175–95.15. Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996; 62:155–69.
Article16. Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article17. Green LC, Wagner DA, Glogoski J, et al. Analysis of nitrate, nitrite and [15N]nitrate in biologic fluids. Anal Biochem. 1982; 126:131–8.18. Beauchamp C, Fridovich L. Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem. 1971; 44:276–87.19. Teufelhofer O, Weiss R-M, Parzefall W, et al. Promyelocytic HL60 cells express NADPH oxidase and are excellent targets in a rapid spectrophotometric microplate assay for extracellular aberrations. Toxicolol Sci. 2003; 76:376–93.20. Joseph JA, Wang H. Quantifying cellular oxidative stress by di-chlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999; 27:612–16.21. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–42.22. Kim KO, Yoon TJ, Choi GJ. Induction of angiogenic cytokines in cultured RPE by oxidative stress. J Korean Ophthalmol Soc. 2004; 45:1742–9.23. Chakravarthy U, Hayes RG, Stitt AW, et al. Constitutive nitric oxide synthase expression in retinal vascular endothelial cells is suppressed by high glucose and advanced glycation end products. Diabetes. 1998; 47:945–52.
Article24. Seigel GM, Chiu L, Paxhia A. Inhibition of neuroretinal cell death by insulinlike growth factor-1 and its analogs. Mol Vis. 2000; 6:157–63.25. Barber AJ, Nakamura M, Wolpert EB, et al. Insulin rescues retinal neurons from apoptosis by a phosphatidyl 3-kinase/Akt-mediated mechanism that reduces the activation of Caspases-3. J Biol Chem. 2001; 276:32814–21.26. Nakamura M, Barber AJ, Antonetti DA, et al. Excessive hexos-amines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. J Biol Chem. 2001; 276:43748–55.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Glucose Concentrations on Reactive Oxygen production and Cellular Activity in Retinal Pigment Epithelial Cells
- Effect of High Glucose on the Production of Reactive Oxygen Species in R28 Cells
- Effect of High Glucose on the Production of Reactive Oxygen Species in Trabecular Meshwork Cells
- The Effect of Simvastatin on the Expression of Catalase in Human Retinal Pigment Epithelial Cells
- Cytoprotective Effect of Polyphenolic Compounds against Oxidative Stress in Cultured Retinal Pigment Epithelial Cells