J Korean Surg Soc.
2011 Sep;81(3):163-168. 10.4174/jkss.2011.81.3.163.
Clinical outcomes of TS-1 chemotherapy for advanced and recurrent gastric cancer
- Affiliations
-
- 1Department of Surgery, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. ch63.yoo@samsung.com
- KMID: 2212193
- DOI: http://doi.org/10.4174/jkss.2011.81.3.163
Abstract
- PURPOSE
Titanium silicate (TS)-1 chemotherapy has been widely used against gastric cancer in Japan. The aim of the present study was to assess the efficacy and hematological safety of TS-1 as treatment for advanced and recurrent gastric cancer.
METHODS
From September 2006 to February 2011, 51 advanced or recurrent gastric cancers were treated with TS-1. One course of treatment consisted of 40, 50, or 60 mg/m2 of TS-1 twice a day for 28 days, followed by withdrawal for two weeks. The primary end point was progression-free survival (PFS), and the secondary end point was overall survival (OS).
RESULTS
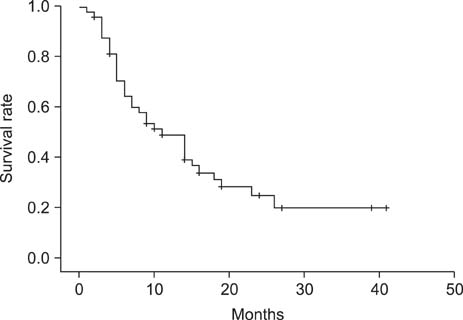
The disease control rate was 39.2% (complete response, 0/51; partial response, 6/51; stable disease, 14/51; progressive disease, 23/51; not evaluable, 8/51). The median PFS was 4.0 months (95% confidence interval [CI], 2.2 to 5.7); the median PFS of the advanced group was 6.0 months (95% CI, 2.8 to 9.1), and the median PFS of the recurrent group was 3.0 months (95% CI, 1.8 to 4.1). The median OS was 11.0 months (95% CI, 6.3 to 15.6); the median OS of the advanced group was 10.0 months (95% CI, 4.9 to 15.0), and the median OS of the recurrent group was 14.0 months (95% CI, 4.1 to 23.8). Grade 3 or 4 hematological toxicity occurred in three patients (5.9%), anemia occurred in two patients (3.9%), and thrombocytopenia occurred in one patient (2%).
CONCLUSION
TS-1 chemotherapy was safe and effective, with relatively long PFS and OS in patients with advanced and recurrent gastric cancers.
MeSH Terms
Figure
Reference
-
1. Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev. 2000. 26:243–255.2. Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998. 34:1715–1720.3. Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology. 2000. 58:191–197.4. Shirasaka T, Nakano K, Takechi T, Satake H, Uchida J, Fujioka A, et al. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996. 56:2602–2606.5. Kondo K, Murase M, Kodera Y, Akiyama S, Ito K, Yokoyama Y, et al. Feasibility study on protracted infusional 5-fluorouracil and consecutive low-dose cisplatin for advanced gastric cancer. Oncology. 1996. 53:64–67.6. Chung YS, Yamashita Y, Inoue T, Matsuoka T, Nakata B, Onoda N, et al. Continuous infusion of 5-fluorouracil and low dose cisplatin infusion for the treatment of advanced and recurrent gastric adenocarcinoma. Cancer. 1997. 80:1–7.7. Kim R, Murakami S, Ohi Y, Inoue H, Yoshida K, Toge T. A phase II trial of low dose administration of 5-fluorouracil and cisplatin in patients with advanced and recurrent gastric cancer. Int J Oncol. 1999. 15:921–926.8. Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000. 18:2648–2657.9. Ohtsu A, Shimada Y, Shirao K, Boku N, Hyodo I, Saito H, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol. 2003. 21:54–59.10. Wils J. The treatment of advanced gastric cancer. Semin Oncol. 1996. 23:397–406.11. Wils JA, Klein HO, Wagener DJ, Bleiberg H, Reis H, Korsten F, et al. Sequential high-dose methotrexate and fluorouracil combined with doxorubicin--a step ahead in the treatment of advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cooperative Group. J Clin Oncol. 1991. 9:827–831.12. Ohtsu A, Shimada Y, Yoshida S, Saito H, Seki S, Morise K, et al. Phase II study of protracted infusional 5-fluorouracil combined with cisplatinum for advanced gastric cancer: report from the Japan Clinical Oncology Group (JCOG). Eur J Cancer. 1994. 30A:2091–2093.13. Katai H, Maruyama K, Sasako M, Sano T, Okajima K, Kinoshita T, et al. Mode of recurrence after gastric cancer surgery. Dig Surg. 1994. 11:99–103.14. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000. 87:236–242.15. Lehnert T, Rudek B, Kienle P, Buhl K, Herfarth C. Impact of diagnostic laparoscopy on the management of gastric cancer: prospective study of 120 consecutive patients with primary gastric adenocarcinoma. Br J Surg. 2002. 89:471–475.16. Kobayashi O, Konishi K, Kanari M, Cho H, Yoshikawa T, Tsuburaya A, et al. Unusual survival for more than 2 years with peritoneal metastases of gastric cancer. Gastric Cancer. 2002. 5:47–50.17. Fujitani K, Tsujinaka T, Hirao M. Feasibility study of S-1 for resectable gastric cancer with peritoneal seeding. Hepatogastroenterology. 2003. 50:889–892.18. Mori T, Fujiwara Y, Yano M, Tamura S, Yasuda T, Takiguchi S, et al. Experimental study to evaluate the usefulness of S-1 in a model of peritoneal dissemination of gastric cancer. Gastric Cancer. 2003. 6:Suppl 1. 13–18.19. Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. Predictive value of chemotherapy-induced neutropenia for the efficacy of oral fluoropyrimidine S-1 in advanced gastric carcinoma. Br J Cancer. 2007. 97:37–42.20. Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. Analysis of risk factors for severe adverse effects of oral 5-fluorouracil S-1 in patients with advanced gastric cancer. Gastric Cancer. 2007. 10:129–134.21. Kim HO, Hwang SI, Hong HP, Yoo CH. Radiofrequency ablation for metachronous hepatic metastases from gastric cancer. Surg Laparosc Endosc Percutan Tech. 2009. 19:208–212.22. Takagi T, Nakase Y, Fukumoto K, Miyagaki T, Ishida E, Kobayashi Y, et al. Long-term disease-free survival following multimodal treatment in a patient with curatively unresectable advanced gastric cancer with metachronous liver metastasis. Gan To Kagaku Ryoho. 2010. 37:2421–2423.23. Kunieda K, Saji S, Sugiyama Y, Osada S, Sano J, Nagao N, et al. Evaluation of treatment for synchronous hepatic metastases from gastric cancer with special reference to long-term survivors. Surg Today. 2002. 32:587–593.24. Yoshida M, Ohtsu A, Boku N, Miyata Y, Shirao K, Shimada Y, et al. Long-term survival and prognostic factors in patients with metastatic gastric cancers treated with chemotherapy in the Japan Clinical Oncology Group (JCOG) study. Jpn J Clin Oncol. 2004. 34:654–659.25. Rougier P, Ducreux M, Mahjoubi M, Pignon JP, Bellefqih S, Oliveira J, et al. Efficacy of combined 5-fluorouracil and cisplatinum in advanced gastric carcinomas. A phase II trial with prognostic factor analysis. Eur J Cancer. 1994. 30A:1263–1269.26. Louvet C, Carrat F, Mal F, Mabro M, Beerblock K, Vaillant JC, et al. Prognostic factor analysis in advanced gastric cancer patients treated with hydroxyurea, leucovorin, 5-fluorouracil, and cisplatin (HLFP regimen). Cancer Invest. 2003. 21:14–20.27. Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer: pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004. 22:2395–2403.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety Profile of TS-1 or TS-1/CDDP in Patients with Advanced Gastric Cancer
- Neoadjuvant Chemotherapy in Asian Patients With Locally Advanced Gastric Cancer
- Preoperative Chemotherapy in Advanced Stomach Cancer (Cons)
- 5-Fluorouracil, heptaplatin and UFT combination chemotherapy for advanced or recurrent gastric cancer
- Preoperative Chemotherapy in Advanced Stomach Cancer (Pros)