J Korean Acad Periodontol.
2009 Sep;39(3):331-337. 10.5051/jkape.2009.39.3.331.
Induction of IL-8 and reactive oxygen species in periodontal ligament cells by Aggregatibacter actinomycetemcomitans
- Affiliations
-
- 1Department of Oral Biology, College of Dentistry, Yonsei University, Seoul, Korea. yu618@yuhs.ac
- 2Department of Applied Life Science, The Graduate School, Yonsei University, Seoul, Korea.
- 3BK21 project, Oral Science Research Center, and Research Center for Orofacial Hard Tissue Regeneration, College of Dentistry, Yonsei University, Seoul, Korea.
- KMID: 2212147
- DOI: http://doi.org/10.5051/jkape.2009.39.3.331
Abstract
- PURPOSE
Interleukin (IL)-8 is one of pro-inflammatory cytokines. Reactive oxygen species (ROS) are reduced metabolites of O2. Aggregatibacter actinomycetemcomitans is one of representative periodontopathogens. To investigate the role of A. actinomycetemcomitans in IL-8 expression of periodontal ligament (PDL) cells, we estimated the production of IL-8 and ROS in A. actinomycetemcomitans treated PDL cells.
METHODS
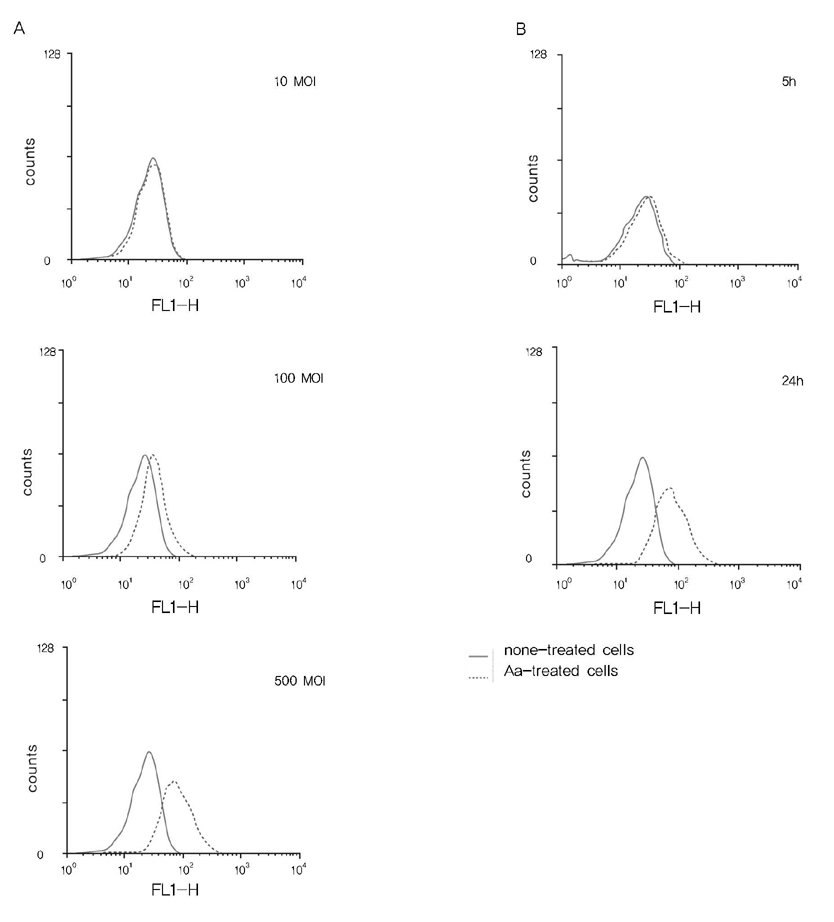
The IL-8 production was determined by enzyme-linked immunosorbent assay. The ROS production was estimated using H2DCFDA and FACS.
RESULTS
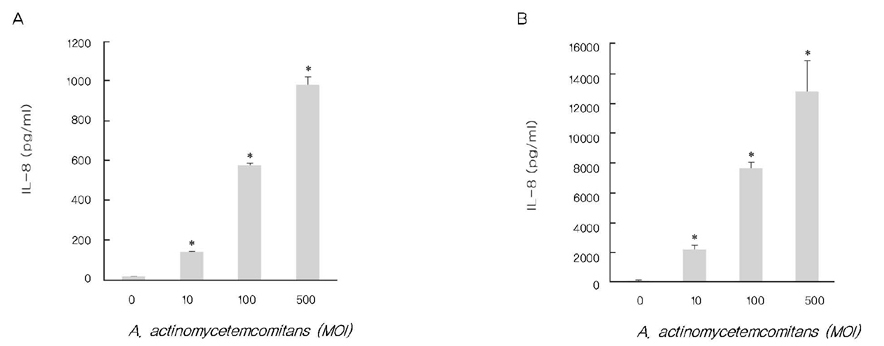
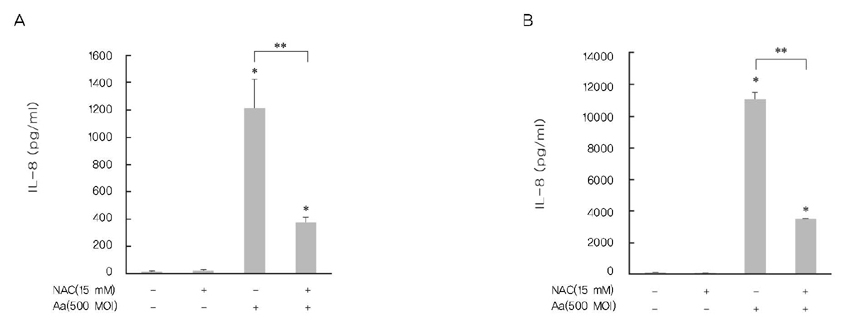
A. actinomycetemcomitans increased the production of IL-8 and ROS at 10, 100, and 500 multiplicity of infection. N-cetylcysteine, an antioxidant of ROS, down-regulated the production of IL-8 induced by A. actinomycetemcomitans.
CONCLUSION
These results suggest that A. actinomycetemcomitans induces IL-8 production and ROS may act as a mediator in this process.
Keyword
MeSH Terms
Figure
Reference
-
1. Ishikawa I. Host responses in periodontal diseases: a preview. Periodontol 2000. 2007. 43:9–13.
Article2. Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992. 307:97–101.
Article3. Tanaka S. Signaling axis in osteoclast biology and therapeutic targeting in the RANKL/RANK/OPG system. Am J Nephrol. 2007. 27:466–478.
Article4. Bendre MS, Montague DC, Peery T, et al. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003. 33:28–37.
Article5. Bendre MS, Margulies AG, Walser B, et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-κB ligand pathway. Cancer Res. 2005. 65:11001–11009.
Article6. Tsai CC, Ho YP, Chen CC. Levels of interleukin-1 β and interleukin-8 in gingival crevicular fluids in adult periodontitis. J Periodontol. 1995. 66:852–859.
Article7. Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Levels of interleukin-1 β, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol. 2000. 71:1535–1545.
Article8. Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Characterization of cellular infiltrate, detection of chemokine receptor CCR5 and interleukin-8 and RANTES chemokines in adult periodontitis. J Periodontal Res. 2001. 36:194–203.
Article9. Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005. 38:135–187.
Article10. Yamaji Y, Kubota T, Sasaguri K, et al. Inflammatory cytokine gene expression in human periodontal ligament fibroblasts stimulated with bacterial lipopolysaccharides. Infect Immun. 1995. 63:3576–3581.
Article11. Yamamoto T, Kita M, Oseko F, et al. Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J Periodontal Res. 2006. 41:554–559.
Article12. Lee SH, Kim KK, Choi BK. Upregulation of intercellular adhesion molecule 1 and proinflammatory cytokines by the major surface proteins of Treponema maltophilum and Treponema lecithinolyticum, the phylogenetic group IV oral spirochetes associated with periodontitis and endodontic infections. Infect Immun. 2005. 73:268–276.
Article13. Patil C, Rossa C Jr, Kirkwood KL. Actinobacillus actinomycetemcomitans lipopolysaccharide induces interleukin-6 expression through multiple mitogen-activated protein kinase pathways in periodontal ligament fibroblasts. Oral Microbiol Immunol. 2006. 21:392–398.
Article14. Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002. 82:47–95.
Article15. Lakshminarayanan V, Beno DW, Costa RH, Roebuck KA. Differential regulation of interleukin-8 and intercellular adhesion molecule-1 by H2O2 and tumor necrosis factor-α in endothelial and epithelial cells. J Biol Chem. 1997. 272:32910–32918.
Article16. Verhasselt V, Goldman M, Willems F. Oxidative stress up-regulates IL-8 and TNF-α synthesis by human dendritic cells. Eur J Immunol. 1998. 28:3886–3890.
Article17. Josse C, Boelaert JR, Best-Belpomme M, Piette J. Importance of post-transcriptional regulation of chemokine genes by oxidative stress. Biochem J. 2001. 360:321–333.
Article18. Hwang YS, Jeong M, Park JS, et al. Interleukin-1α stimulates IL-8 expression through MAP kinase and ROS signaling in human gastric carcinoma cells. Oncogene. 2004. 23:6603–6611.
Article19. Pelaia G, Cuda G, Vatrella A, et al. Effects of hydrogen peroxide on MAPK activation, IL-8 production and cell viability in primary cultures of human bronchial epithelial cells. J Cell Biochem. 2004. 93:142–152.
Article20. Lee YS, Bak EJ, Kim M, et al. Induction of IL-8 in periodontal ligament cells by H2O2. J Microbiol. 2008. 46:579–584.21. Seo JY, Kim H, Kim KH. Transcriptional regulation by thiol compounds in Helicobacter pylori-induced interleukin-8 production in human gastric epithelial cells. Ann N Y Acad Sci. 2002. 973:541–545.
Article22. Christersson LA, Albini B, Zambon JJ, Wikesjo UM, Genco RJ. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. I. Light, immunofluorescence and electron microscopic studies. J Periodontol. 1987. 58:529–539.
Article23. Christersson LA, Wikesjo UM, Albini B, Zambon JJ, Genco RJ. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. II. Correlation between immunofluorescence and culture techniques. J Periodontol. 1987. 58:540–545.
Article24. Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997. 336:1066–1071.
Article25. Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-κ B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. Embo J. 1993. 12:2005–2015.
Article26. DeForge LE, Preston AM, Takeuchi E, et al. Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem. 1993. 268:25568–25576.
Article27. O'Hara AM, Bhattacharyya A, Bai J, et al. Tumor necrosis factor (TNF)-α-induced IL-8 expression in gastric epithelial cells: role of reactive oxygen species and AP endonuclease-1/redox factor (Ref)-1. Cytokine. 2009. 46:359–369.28. Kim do Y, Jun JH, Lee HL, et al. N-acetylcysteine prevents LPS-induced pro-inflammatory cytokines and MMP2 production in gingival fibroblasts. Arch Pharm Res. 2007. 30:1283–1292.
Article29. Di Paola R, Mazzon E, Zito D, et al. Effects of Tempol, a membrane-permeable radical scavenger, in a rodent model periodontitis. J Clin Periodontol. 2005. 32:1062–1068.
Article30. Toker H, Ozdemir H, Eren K, Ozer H, Sahin G. N-acetylcysteine, a thiol antioxidant, decreases alveolar bone loss in experimental periodontitis in rats. J Periodontol. 2009. 80:672–678.
Article31. Long P, Hu J, Piesco N, Buckley M, Agarwal S. Low magnitude of tensile strain inhibits IL-1β-dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. J Dent Res. 2001. 80:1416–1420.
Article32. Lee HJ, Cho JW, Kim SC, et al. Roles of p38 and ERK MAP kinases in IL-8 expression in TNF-α- and dexamethasone-stimulated human periodontal ligament cells. Cytokine. 2006. 35:67–76.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nuclear Factor-kappaB Dependent Induction of TNF-alpha and IL-1beta by the Aggregatibacter actinomycetemcomitans Lipopolysaccharide in RAW 264.7 Cells
- Antibacterial effect of genistein against periodontal pathogens
- Aggregatibacter actinomycetemcomitans Strongly Stimulates Endothelial Cells to Produce Monocyte Chemoattractant Protein-1 and Interleukin-8
- Induction of osteoclastogenesis-inducing cytokines and invasion by alive Aggregatibacter actinomycetemcomitans in osteoblasts
- The effect of rosehip extract on TNF-α, IL-1β, and IL-8 production in THP-1-derived macrophages infected with Aggregatibacter actinomycetemcomitans