Clinical Practice Guideline for Adequate Diagnosis and Effective Treatment of Gastrointestinal Stromal Tumor in Korea
- Affiliations
-
- 1Devision of Oncology / Department of Internal Medicine, Ulsan University College of Medicine, Korea. ykkang@amc.seoul.kr
- KMID: 2184924
- DOI: http://doi.org/10.5124/jkma.2007.50.9.830
Abstract
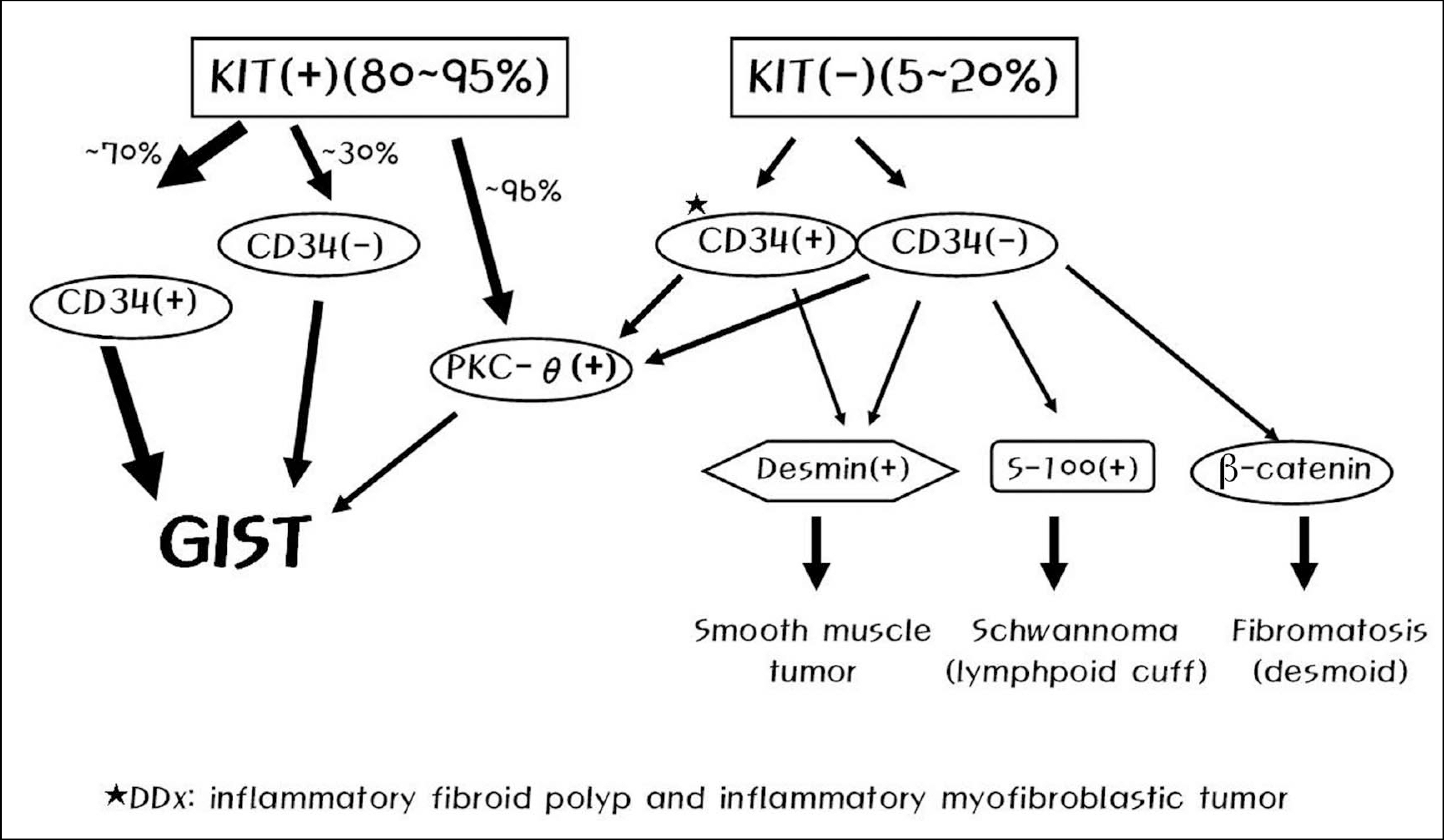
- Despite the rarity in incidence and prevalence, gastrointestinal stromal tumor (GIST) has emerged as a distinct pathogenetic entity, and the clinical management of GIST has been evolving very rapidly due to the recent recognition of its oncogenic signal transduction pathway and the introduction of new molecular-targeted therapy. Successful management of GIST in localized and advanced stages requires a multidisciplinary approach firmly based on accurate histopathologic diagnosis. However, standardized guidelines for the management of Korean GIST patients do not exist. This study was performed to provide a guideline for standardized diagnosis and treatment for GIST in Korea. Expert panel members of the Korean GIST Study Group (KGSG) thoroughly reviewed the relevant literature including European Society of Medical Oncology and National Comprehensive Cancer Network guidelines and shared their experience and opinions to make a consensus on twenty-five topics related with pathologic diagnosis, surgical management, and medical treatment of GIST. The consensus described in this article was presented as the basis for a guideline of diagnosis and treatment for patients with GIST that would be used to facilitate the optimal clinical practice in Korea.
MeSH Terms
Figure
Cited by 3 articles
-
Clinical Practice Guideline for Accurate Diagnosis and Effective Treatment of Gastrointestinal Stromal Tumor in Korea
Yoon-Koo Kang, Kyoung-Mee Kim, Taesung Sohn, Dongil Choi, Hye Jin Kang, Min-Hee Ryu, Woo Ho Kim, Han-Kwang Yang
J Korean Med Sci. 2010;25(11):1543-1552. doi: 10.3346/jkms.2010.25.11.1543.Endoscopic Full-Thickness Resection for Gastric Subepithelial Lesions Arising from the Muscularis Propria
Ah Lon Jung, Sang Wook Park, Gun Young Hong, Hyeong Chul Moon, Seo Joon Eun
Clin Endosc. 2021;54(1):131-135. doi: 10.5946/ce.2020.070.Clinical Practice Guideline for Accurate Diagnosis and Effective Treatment of Gastrointestinal Stromal Tumor in Korea
Yoon-Koo Kang, Hye Jin Kang, Kyoung-Mee Kim, Taesung Sohn, Dongil Choi, Min-Hee Ryu, Woo Ho Kim, Han-Kwang Yang,
Cancer Res Treat. 2012;44(2):85-96. doi: 10.4143/crt.2012.44.2.85.
Reference
-
References
1. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors. A consensus approach. Hum Pathol. 2002; 33:459–465.
Article2. Kim KM, Kang DW, Moon WS, Park JB, Park CK, Sohn JH, Jeong JS, Cho MY, Jin SY, Choi JS, Kang DY. Gastrointestinal stromal tumors in Koreans: It's incidence and the clinical, pathologic and immunohistochemical findings. J Korean Med Sci. 2005; 20:977–984.
Article3. Miettinen M, Lasota J. Gastrointestinal stromal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006; 130:1466–1478.
Article4. Kim KM, Kang DW, Moon WS, Park JB, Park CK, Sohn JH, Jeong JS, Cho MY, Jin SY, Choi JS, Kang DY. PKC?theta expression in gastrointestinal stromal tumor. Mod Pathol. 2006; 19:1480–1486.5. Miettinen MM, Sarlomo?Rikala M, Kovatich AJ, Lasota J. Calponin and h?caldesmon in soft tissue tumors: Consistent h?caldesmon immunoreactivity in gastrointestinal stromal tumors indicates traits of smooth muscle differentiation. Mod Pathol. 1999; 12:756–762.6. Hornick JL, Fletcher CD. The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol. 2007; 38:679–687.
Article7. Townsend. CM, ed. Sabiston textbook of surgery: The biological basis of modern surgical practice. 17th ed.Philadelphia, W.B. Saunders Co.;2004.8. Brunicardi FC, ed. Schwartz's principles of surgery. 8th ed.New York: McGraw?Hill, Medical Publishing Division;2005.9. Trent JC, Benjamin RS. New developments in gastrointestinal stromal tumor. Curr Opin Oncol. 2006; 18:386–395.
Article10. Blay JY, Bonvalot S, Casali P, Choi H, Debiec?Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PCW, Joensuu H, Le Cesne A, Mac Clure J, Maurel J, Nupponen N, Ray? Coquard I, Reichardt P, Sciot R, Stroobants S, van Glabbeke M, van Oosterom A, Demetri GD. Consensus meeting for the management of gastrointestinal stromal tumors: Report of the GIST consensus conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol. 2005; 16:566–578.
Article11. Gold JS, Dematteo RP. Combined surgical and molecular therapy: The gastrointestinal stromal tumor model. Ann Surg. 2006; 244:176–184.12. Iwahashi M, Takifuji K, Ojima T, Nakamura M, Nakamori M, Nakatani Y, Ueda K, Ishida K, Naka T, Ono K, Yamaue H. Surgical management of small gastrointestinal stromal tumors of the stomach. World J Surg. 2006; 30:28–35.
Article13. Otani Y, Furukawa T, Yoshida M, Saikawa Y, Wada N, Ueda M, Kubota T, Mukai M, Kameyama K, Sugino Y, Kumai K, Kitajima M. Operative indications for relatively small (2∼5cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery. 2006; 139:484–492.14. Choi SM, Kim MC, Jung GJ, Kim HH, Kwon HC, Choi SR, Jang JS, Jeong JS. Laparoscopic wedge resection for gastric GIST; long?term follow?up results. Eur J Surg Oncol. 2007; 33:444–447.
Article15. Novitsky YW, Kercher KW, Sing RF, Heniford BT. Long?term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006; 243:738–745. ; discussion 745–737.
Article16. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: Recurrence patterns and prognostic factors for survival. Ann Surg. 2000; 231:51–58.17. Raut CP, Posner M, Desai J, Morgan JA, George S, Zahrieh D, Fletcher CD, Demetri GD, Bertagnolli MM. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006; 24:2325–2331.
Article18. DeMatteo RP, Owzar K, Maki RG, Pisters PW, Blackstein M, Antonescu CR, Blanke C, Demetri G, Von Mehren M, Ballman K, and the American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvnat GIST Study Team. Adjuvant imatinib mesylate increases recurrence free survival (RFS) in patients with completely resected localized primary gastrointestinal stromal tumor (GIST): North American Iintergroup phase III trial ACOSGO Z9001. J Clin Oncol (Meeting Abstracts). 2007; 25:abstr 10079.19. Kim TW, Lee H, Kang YK, Choe MS, Ryu MH, Chang HM, Kim JS, Yook JH, Kim BS, Lee JS. Prognostic significance of c?KIT mutation in localized gastrointestinal stromal tumors. Clin Cancer Res. 2004; 10:3076–3081.
Article20. Bumming P, Andersson J, Meis?Kindblom JM, Klingenstierna H, Engstrom K, Stierner U, Wangberg B, Jansson S, Ahlman H, Kindblom LG, Nilsson B. Neoadjuvant, adjuvant and palliative treatment of gastrointestinal stromal tumours (GIST) with imatinib: A centre?based study of 17 patients. Br J Cancer. 2003; 89:460–464.
Article21. Loughrey MB, Mitchell C, Mann GB, Michael M, Waring PM. Gastrointestinal stromal tumour treated with neoadjuvant imatinib. J Clin Pathol. 2005; 58:779–781.
Article22. Oh JS, Lee J?L, Kim M?J, Ryu M?H, Chang HM, Kim T?W, Jansg SJ, Yook JH, Oh ST, Kim BS, Kang YK. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors of the stomach: Report of three cases. Cancer Res Treat. 2006; 38:178–183.23. DeMatteo RP, Heinrich MC, El?Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: Before and after STI?571. Hum Pathol. 2002; 33:466–477.
Article24. Rankin C, Von Mehren M, Blanke C, Benjamin R, Fletcher CDM, Bramwell V, Crowley J, Borden E, Demetri GD. Dose effect of imatinib (im) in patients (pts) with metastatic GIST ? phase III sarcoma group study S0033. J Clin Oncol (Meeting Abstracts). 2004; 22:9005.
Article25. Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, Bertulli R, Judson I. Progression?free survival in gastrointestinal stromal tumours with high?dose imatinib: Randomised trial. Lancet. 2004; 364:1127–1134.
Article26. Debiec?Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, Blay J?Y, Leyvraz S, Stul M, Casali PG, Zalcberg J, Verweij J, Van Glabbeke M, Ha-gemeijer A, Judson I. Kit mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006; 42:1093–1103.27. Lee J?L, Ryu M?H, Chang HM, Kim TW, Kang HJ, Sohn HJ, Lee JS, Kang Y?K. Clinical outcome in gastrointestinal stromal tumor patients who interrupted imatinib after achieving stable disease or better response. Jpn J Clin Oncol. 2006; 36:704–711.28. Blay J?Y. Le Cesne A, Ray?Coquard I, Bui B, Duffaud F, Delbaldo C, Adenis A, Viens P, Rios M, Bompas E, Cupissol D, Guillemet C, Kerbrat P, Fayette J, Chabaud S, Berthaud P, Perol D. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol. 2007; 25:1107–1113.29. Ryu M?H, Lee J?L, Chang HM, Kim TW, Kang HJ, Sohn HJ, S . LJ, Kang Y?K. Patterns of progression in gastrointestinal stromal tumor treated with imatinib mesylate. Jpn J Clin Oncol. 2006; 36:17–24.30. Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, Martens M, van den Borne B, Cole P, Sciot R. 18FDG?positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (glivec?). Eur J Cancer. 2003; 39:2012–2020.31. Jager PL, Gietema JA, van der Graaf WT. Imatinib mesylate for the treatment of gastrointestinal stromal tumours: Best monitored with FDG PET. Nucl Med Commun. 2004; 25:433–438.
Article32. Antoch G, Kanja J, Bauer S, Kuehl H, Renzing?Koehler K, Schuette J, Bockisch A, Debatin JF, Freudenberg LS. Comparison of PET, CT, and dual?modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004; 45:357–365.33. Linton KM, Taylor MB, Radford JA. Response evaluation in gastrointestinal stromal tumours treated with imatinib: Misdiagnosis of disease progression on CT due to cystic change in liver metastases. Br J Radiol. 2006; 79:e40–44.
Article34. Choi H, Charnsangavej C, Faria SdC, Tamm EP, Benjamin RS, Johnson MM, Macapinlac HA, Podoloff DA. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: A quantitative analysis correlated with FDG PET findings. Am J Roentgenol. 2004; 183:1619–1628.35. Desai J, Shankar S, Heinrich MC, Fletcher JA, Fletcher CDM, Tuncali K, Silverman SG, Van den Abbeele AD, VanSonnenberg E, Demetri GD. Clonal evolution of resistance to imatinib (im) in patients (pts) with gastrointestinal stromal tumor (GIST): Molecular and radiologic evaluation of new lesions. J Clin Oncol (Meeting Abstracts). 2004; 23:197 (abstr 3010).
Article36. Bauer S, Hart J, de Wit M, Lang H, Grabellus F, Antoch G, Niebel W, Erhard J, Ebeling P, Zeth M, Taeger G, Seeber S, Flasshove M, Schutte J. Resection of residual disease in patients with metastatic gastrointestinal stromal tumors responding to treatment with imatinib. Int J Cancer. 2005; 117:316–325.
Article37. Dileo P, Randhawa R, Vansonnenberg E, Shankar S, Desai J, Morgan JA, Tuncali K, Van Den Abbeele A, Silverman SG, Demetri GD. Safety and efficacy of percutaneous radio? frequency ablation (RFA) in patients (pts) with metastatic gastrointestinal stromal tumor (GIST) with clonal evolution of lesions refractory to imatinib mesylate (im). J Clin Oncol (Meeting Abstracts). 2004; 22:9024.38. Zalcberg JR, Verweij J, Casali PG, Le Cesne A, Reichardt P, Blay JY, Schlemmer M, Van Glabbeke M, Brown M, Judson IR. Outcome of patients with advanced gastro?intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400mg. Eur J Cancer. 2005; 41:1751–1757.39. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006; 368:1329–1338.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Practice Guideline for Accurate Diagnosis and Effective Treatment of Gastrointestinal Stromal Tumor in Korea
- Clinical Practice Guideline for Accurate Diagnosis and Effective Treatment of Gastrointestinal Stromal Tumor in Korea
- Incidental Gastrointestinal Subepithelial Mass
- Diagnosis of a Gastrointestinal Stromal Tumor Presenting as a Prostatic Mass: A Case Report
- A Case of Massive Bleeding from Jejunal Stromal Tumor Diagnosed by Intraoperative Enteroscopy: A Case of Jejunal Stromal Tumor Bleeding