J Gynecol Oncol.
2012 Jan;23(1):5-10. 10.3802/jgo.2012.23.1.5.
Comparison of the Seeplex HPV4A ACE and the Cervista HPV assays for the detection of HPV in hybrid capture 2 positive media
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea. jklee38@gmail.com
- 2Department of Obstetrics and Gynecology, Kangbuk Samsung Hospital, Seoul, Korea.
- 3Department of Pathology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- KMID: 2177507
- DOI: http://doi.org/10.3802/jgo.2012.23.1.5
Abstract
OBJECTIVE
To validate the efficacy of Seeplex HPV4A ACE for the detection of high-risk (HR) human papillomavirus (HPV) and HPV 16 and/or HPV 18 genotypes as compared to the PCR method and the Cervista HPV assays in cervical swab samples.
METHODS
Besides liquid-based cytology, additional 97 cervical swab samples were collected for HPV genotyping by HPV4A ACE, Cervista HPV assays, and PCR method. To check the statistical differences, we also conducted the paired proportion test, Cohen's kappa statistic, and a receiver operating characteristic curve.
RESULTS
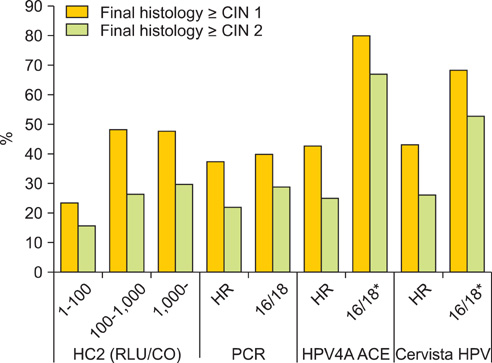
Seeplex HPV4A ACE and the Cervista HPV HR showed substantial agreement with PCR for detection of HR HPVs (88.3%, kappa=0.767 and 81.7%, kappa=0.636, respectively). Seeplex HPV4A ACE also showed substantial agreement with the Cervista HPV 16/18 test (89.5%, kappa=0.628). Additionally, the sensitivity and specificity of Seeplex HPV4A ACE and Cervista HPV HR were 91.4% vs. 84.5% and 73.4%, vs. 72.7%, respectively, when those higher than low-grade squamous intraepithelial lesions were regarded as abnormalities. HPV genotyping for HPV 16/18 detected cervical intraepithelial neoplasias (CINs) better than HR HPV tests (66.7% vs. 24.6% by HPV4A ACE, 52.6% vs. 25.9% by Cervista HPV assays in CIN II or more, relatively).
CONCLUSION
Seeplex HPV4A ACE is an effective method as the PCR and the Cervista HPV assays for the detection of HR HPVs and for genotyping of HPV 16 and 18.
MeSH Terms
Figure
Cited by 1 articles
-
Comparison of the PANArray HPV Genotyping Chip Test with the Cobas 4800 HPV and Hybrid Capture 2 Tests for Detection of HPV in ASCUS Women
Eun Young Ki, Yoon Kyung Lee, Ahwon Lee, Jong Sup Park
Yonsei Med J. 2018;59(5):662-668. doi: 10.3349/ymj.2018.59.5.662.
Reference
-
1. Schiffman M, Kjaer SK. Chapter 2: Natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr. 2003. (31):14–19.2. Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005. 6:204.3. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007. 370:890–907.4. Chun JY, Kim KJ, Hwang IT, Kim YJ, Lee DH, Lee IK, et al. Dual priming oligonucleotide system for the multiplex detection of respiratory viruses and SNP genotyping of CYP2C19 gene. Nucleic Acids Res. 2007. 35:e40.5. Day SP, Hudson A, Mast A, Sander T, Curtis M, Olson S, et al. Analytical performance of the Investigational Use Only Cervista HPV HR test as determined by a multi-center study. J Clin Virol. 2009. 45:Suppl 1. S63–S72.6. Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008. 26:Suppl 10. K1–K16.7. Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003. 88:63–73.8. Herrero R, Hildesheim A, Bratti C, Sherman ME, Hutchinson M, Morales J, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000. 92:464–474.9. Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005. 97:1072–1079.10. Galan-Sanchez F, Rodriguez-Iglesias MA. Use of Cervista HPV HR assay for detection of human papillomavirus in samples with hybrid capture borderline negative results. APMIS. 2010. 118:681–684.11. Einstein MH, Martens MG, Garcia FA, Ferris DG, Mitchell AL, Day SP, et al. Clinical validation of the Cervista HPV HR and 16/18 genotyping tests for use in women with ASC-US cytology. Gynecol Oncol. 2010. 118:116–122.12. Ginocchio CC, Barth D, Zhang F. Comparison of the Third Wave Invader human papillomavirus (HPV) assay and the digene HPV hybrid capture 2 assay for detection of high-risk HPV DNA. J Clin Microbiol. 2008. 46:1641–1646.13. Hong JH, Song SH, Kim JK, Han JH, Lee JK. Comparison of the novel human papillomavirus 4 auto-capillary electrophoresis test with the hybrid capture 2 assay and with the PCR HPV typing set test in the detection of high-risk HPV including HPV 16 and 18 genotypes in cervical specimens. J Korean Med Sci. 2009. 24:579–584.14. Iftner T, Germ L, Swoyer R, Kjaer SK, Breugelmans JG, Munk C, et al. Study comparing human papillomavirus (HPV) real-time multiplex PCR and Hybrid Capture II INNO-LiPA v2 HPV genotyping PCR assays. J Clin Microbiol. 2009. 47:2106–2113.15. Mo LZ, Monnier-Benoit S, Kantelip B, Petitjean A, Riethmuller D, Pretet JL, et al. Comparison of AMPLICOR and Hybrid Capture II assays for high risk HPV detection in normal and abnormal liquid-based cytology: use of INNO-LiPA Genotyping assay to screen the discordant results. J Clin Virol. 2008. 41:104–110.16. Baleriola C, Millar D, Melki J, Coulston N, Altman P, Rismanto N, et al. Comparison of a novel HPV test with the Hybrid Capture II (hcII) and a reference PCR method shows high specificity and positive predictive value for 13 high-risk human papillomavirus infections. J Clin Virol. 2008. 42:22–26.17. Ratnam S, Coutlee F, Fontaine D, Bentley J, Escott N, Ghatage P, et al. Clinical performance of the PreTect HPV-Proofer E6/E7 mRNA assay in comparison with that of the Hybrid Capture 2 test for identification of women at risk of cervical cancer. J Clin Microbiol. 2010. 48:2779–2785.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Novel Human Papillomavirus 4 Auto-capillary Electrophoresis Test with the Hybrid Capture 2 Assay and with the PCR HPV Typing Set Test in the Detection of High-Risk HPV Including HPV 16 and 18 Genotypes in Cervical Specimens
- Comparative Analysis of Multiplex PCR and Hybrid Capture System in the Detecttion of Human Papillomavirus in the Uterine Cervix
- Comparison of the AdvanSure Human Papillomavirus Screening Real-Time PCR, the Abbott RealTime High Risk Human Papillomavirus Test, and the Hybrid Capture Human Papillomavirus DNA Test for the Detection of Human Papillomavirus
- Detection of Human Papillomavirus in Cervical Neoplasia using HPVDNAChip(R) and Hybrid capture IITM system
- Clinical Efficacy of High-risk HPV DNA Test Using Hybrid Capture System in Cervical Epithelial Cells