Investig Magn Reson Imaging.
2015 Jun;19(2):88-98. 10.13104/imri.2015.19.2.88.
Measurement of Apparent Diffusion Coefficient Values from Diffusion-Weighted MRI: A Comparison of Manual and Semiautomatic Segmentation Methods
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Seoul, Korea. verocay@snuh.org
- 2Center for Nanoparticle Research, Institute for Basic Science, and School of Chemical and Biological Engineering, Seoul National University, Seoul, Korea.
- 3Department of Internal Medicine, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Radiation Oncology, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2175588
- DOI: http://doi.org/10.13104/imri.2015.19.2.88
Abstract
- PURPOSE
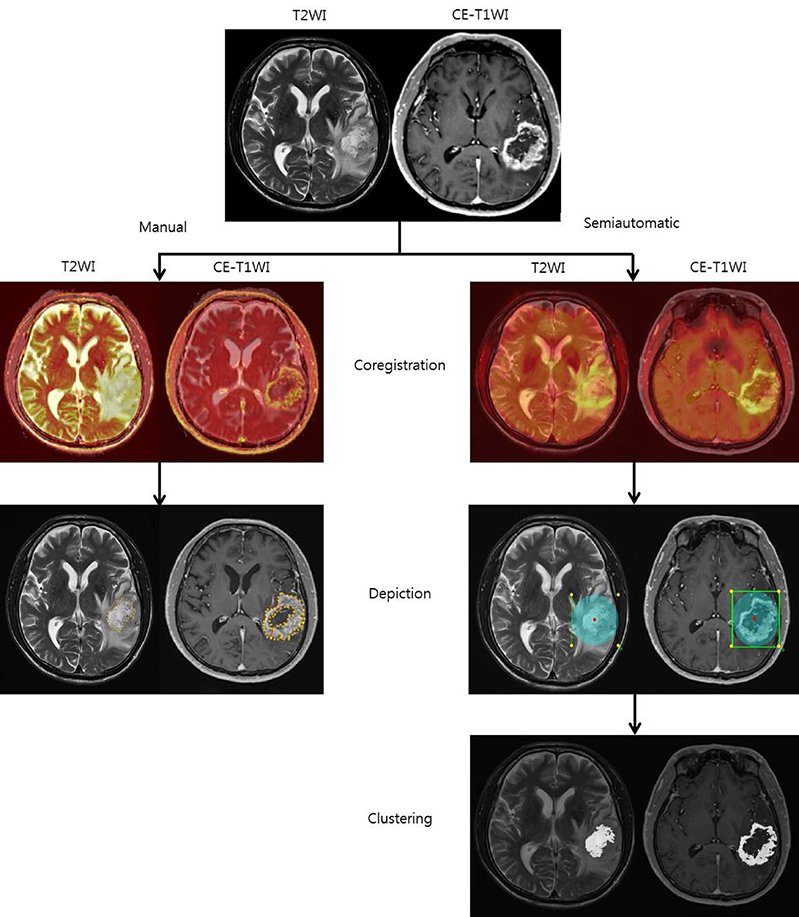
To compare the interobserver and intraobserver reliability of mean apparent diffusion coefficient (ADC) values using contrast-enhanced (CE) T1 weighted image (WI) and T2WI as structural images between manual and semiautomatic segmentation methods.
MATERIALS AND METHODS
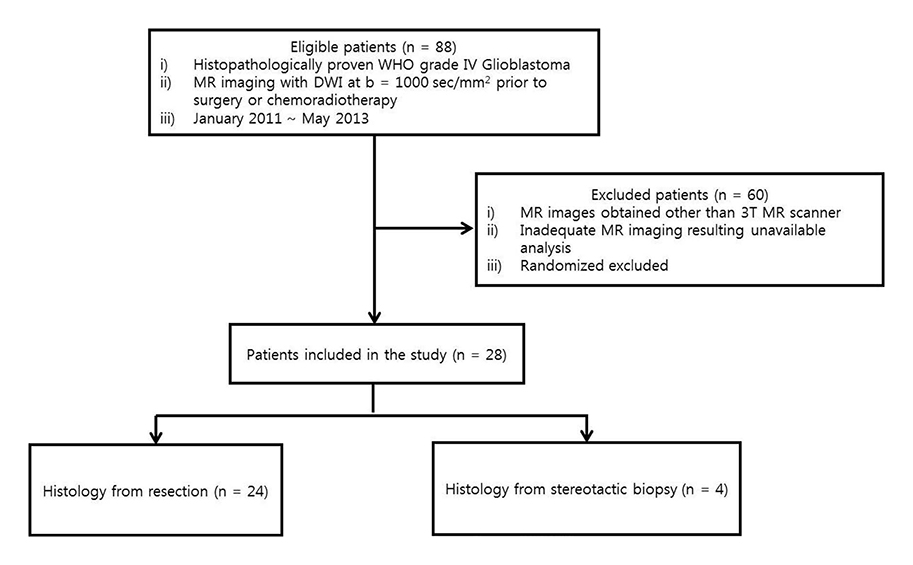
Between January 2011 and May 2013, 28 patients who underwent brain MR with diffusion weighted image (DWI) and were pathologically confirmed as having glioblastoma participated in our study. The ADC values were measured twice in manual and semiautomatic segmentation methods using CE-T1WI and T2WI as structural images to obtain interobserver and intraobserver reliability. Moreover, intraobserver reliabilities of the different segmentation methods were assessed after subgrouping of the patients based on the MR findings.
RESULTS
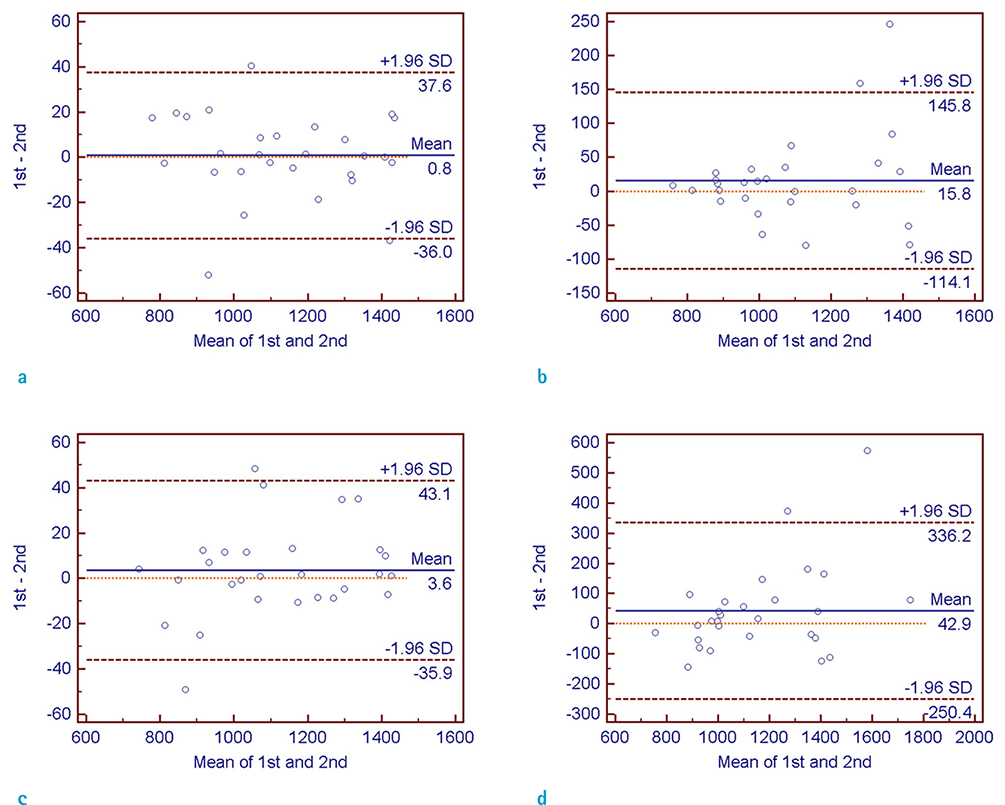
Interobserver and intraobserver reliabilities were high in both manual and semiautomatic segmentation methods on CE-T1WI-based evaluation, while interobserver reliability on T2WI-based evaluation was not high enough to be used in a clinical context. The intraobserver reliability was particularly lower with the T2WI-based semiautomatic segmentation method in the subgroups with involved lobes < or = 2, with partially demarcated tumor borders, poorly demarcated inner margins of the necrotic portion, and with perilesional edema.
CONCLUSION
Both the manual and semiautomatic segmentation methods on CE-T1WI-based evaluation were clinically acceptable in the measurement of mean ADC values with high interobserver and intraobserver reliabilities.
Keyword
Figure
Cited by 1 articles
-
Dynamic Susceptibility Contrast (DSC) Perfusion MR in the Prediction of Long-Term Survival of Glioblastomas (GBM): Correlation with MGMT Promoter Methylation and 1p/19q Deletions
Yong Wonn Kwon, Won-Jin Moon, Mina Park, Hong Gee Roh, Young Cho Koh, Sang Woo Song, Jin Woo Choi
Investig Magn Reson Imaging. 2018;22(3):158-167. doi: 10.13104/imri.2018.22.3.158.
Reference
-
1. Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006; 2:494–503. quiz 491 p following 5162. Law M, Young R, Babb J, Pollack E, Johnson G. Histogram analysis versus region of interest analysis of dynamic susceptibility contrast perfusion MR imaging data in the grading of cerebral gliomas. AJNR Am J Neuroradiol. 2007; 28:761–766.3. Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999; 9:53–60.4. Kang Y, Choi SH, Kim YJ, et al. Gliomas: Histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion-weighted MR imaging--correlation with tumor grade. Radiology. 2011; 261:882–890.5. Provenzale JM, Mukundan S, Barboriak DP. Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology. 2006; 239:632–649.6. Murakami R, Hirai T, Sugahara T, et al. Grading astrocytic tumors by using apparent diffusion coefficient parameters: superiority of a one- versus two-parameter pilot method. Radiology. 2009; 251:838–845.7. Murakami R, Hirai T, Kitajima M, et al. Magnetic resonance imaging of pilocytic astrocytomas: usefulness of the minimum apparent diffusion coefficient (ADC) value for differentiation from high-grade gliomas. Acta Radiol. 2008; 49:462–467.8. Huo J, Okada K, Kim HJ, Pope WB, Goldin JG, Alger JR. CADrx for GBM brain tumors: predicting treatment response from changes in diffusion-weighted MRI. Algorithms. 2009; 2:1350–1367.9. Pope WB, Kim HJ, Huo J, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009; 252:182–189.10. Fletcher-Heath LM, Hall LO, Goldgof DB, Murtagh FR. Automatic segmentation of non-enhancing brain tumors in magnetic resonance images. Artif Intell Med. 2001; 21:43–63.11. Clark MC, Hall LO, Goldgof DB, Velthuizen R, Murtagh FR, Silbiger MS. Automatic tumor segmentation using knowledge-based techniques. IEEE Trans Med Imaging. 1998; 17:187–201.12. Nie J, Xue Z, Liu T, et al. Automated brain tumor segmentation using spatial accuracy-weighted hidden Markov Random Field. Comput Med Imaging Graph. 2009; 33:431–441.13. Kaus MR, Warfield SK, Nabavi A, Black PM, Jolesz FA, Kikinis R. Automated segmentation of MR images of brain tumors. Radiology. 2001; 218:586–591.14. Corso JJ, Sharon E, Dube S, El-Saden S, Sinha U, Yuille A. Efficient multilevel brain tumor segmentation with integrated bayesian model classification. IEEE Trans Med Imaging. 2008; 27:629–640.15. Bjornerud A. The ICE software package: direct co-registration of anatomical and functional datasets using DICOM image geometry information. Proc Hum Brain Mapping. 2003; 19:1018p.16. Sundar H, Shen D, Biros G, Xu C, Davatzikos C. Robust computation of mutual information using spatially adaptive meshes. Med Image Comput Comput Assist Interv. 2007; 10:950–958.17. Pluim JP, Maintz JB, Viergever MA. Mutual-information-based registration of medical images: a survey. IEEE Trans Med Imaging. 2003; 22:986–1004.18. Reed GF, Lynn F, Meade BD. Use of coefficient of variation in assessing variability of quantitative assays. Clin Diagn Lab Immunol. 2002; 9:1235–1239.19. Jung SC, Choi SH, Yeom JA, et al. Cerebral blood volume analysis in glioblastomas using dynamic susceptibility contrast-enhanced perfusion MRI: a comparison of manual and semiautomatic segmentation methods. PLoS One. 2013; 8:e69323.20. Emblem KE, Nedregaard B, Hald JK, Nome T, Due-Tonnessen P, Bjornerud A. Automatic glioma characterization from dynamic susceptibility contrast imaging: brain tumor segmentation using knowledge-based fuzzy clustering. J Magn Reson Imaging. 2009; 30:1–10.21. Prastawa M, Bullitt E, Moon N, Van Leemput K, Gerig G. Automatic brain tumor segmentation by subject specific modification of atlas priors. Acad Radiol. 2003; 10:1341–1348.22. Hsieh TM, Liu YM, Liao CC, Xiao F, Chiang IJ, Wong JM. Automatic segmentation of meningioma from non-contrasted brain MRI integrating fuzzy clustering and region growing. BMC Med Inform Decis Mak. 2011; 11:54.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reversal of a Large Ischemic Lesion with Low Apparent Diffusion Coefficient Value by Rapid Spontaneous Recanalization
- Diffusion-weighted Imaging and Apparent Diffusion Coefficient Maps for the Evaluation of Pyogenic Ventriculitis
- Dysarthria with Hypoglycemia and Reversible Focal Hyperintensity Lesion on Diffusion?Weighted MRI
- SNR and ADC Changes at Increasing b Values among Patients with Lumbar Vertebral Compression Fracture on 1.5T MR Diffusion Weighted Images
- Diffusion-weighted and Dynamic Contrast-enhanced MRI of Metastatic Bone Tumors: Correlation of the Apparent Diffusion Coefficient, K(trans) and ve values