Immune Netw.
2011 Oct;11(5):299-306. 10.4110/in.2011.11.5.299.
Deficiency of Foxp3+ Regulatory T Cells Exacerbates Autoimmune Arthritis by Altering the Synovial Proportions of CD4+ T Cells and Dendritic Cells
- Affiliations
-
- 1Institute of Biomedical Sciences, College of Medicine, Hanyang University, Seoul 133-791, Korea. jhyoun@hanyang.ac.kr
- 2Department of Anatomy & Cell Biology, College of Medicine, Hanyang University, Seoul 133-791, Korea.
- 3Institute of Biomedical Institutes, The Catholic University of Korea, Seoul 137-040, Korea.
- KMID: 2168002
- DOI: http://doi.org/10.4110/in.2011.11.5.299
Abstract
- BACKGROUND
CD4+Fop3+ regulatory T cells (Tregs) are needed to maintain peripheral tolerance, but their role in the development of autoimmune arthritis is still debated. The present study was undertaken to investigate the mechanism by which Tregs influence autoimmune arthritis, using a mouse model entitled K/BxN.
METHODS
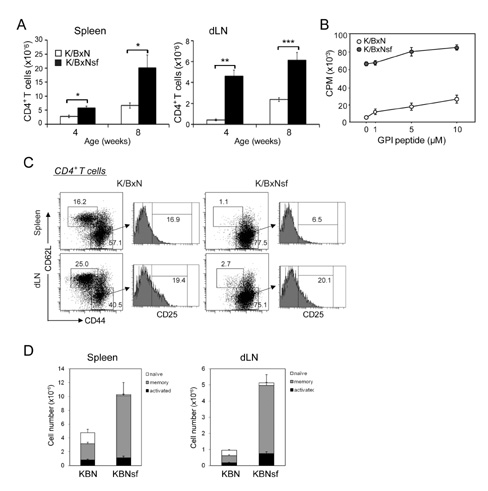
We generated Treg-deficient K/BxNsf mice by congenically crossing K/BxN mice with Foxp3 mutant scurfy mice. The arthritic symptoms of the mice were clinically and histopathologically examined. The proportions and activation of CD4+ T cells and/or dendritic cells were assessed in the spleens, draining lymph nodes and synovial tissue of these mice.
RESULTS
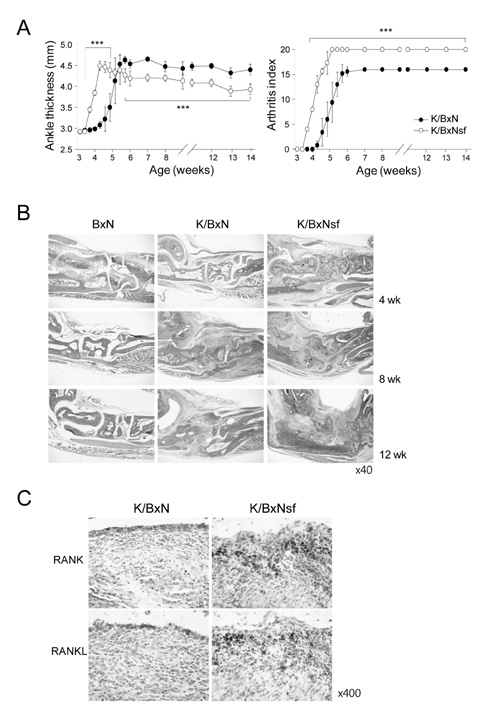
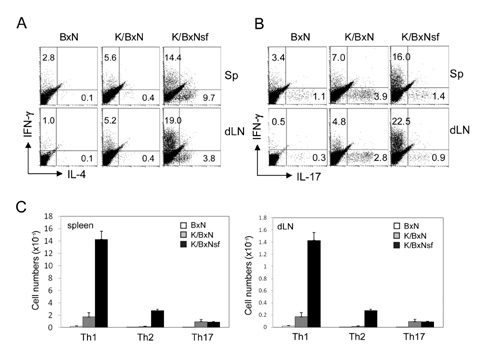
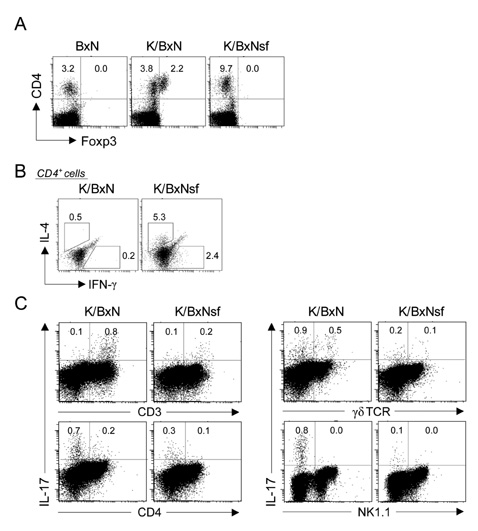
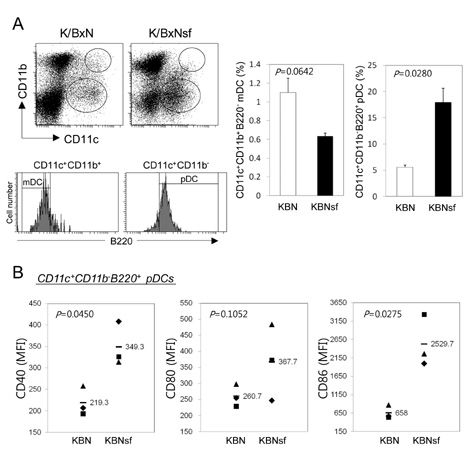
K/BxNsf mice exhibited earlier onset and more aggressive progression of arthritis than their K/BxN littermates. In particular, bone destruction associated with the influx of numerous RANKL+ cells into synovia was very prominent. They also contained more memory phenotype CD4+ T cells, more Th1 and Th2 cells, and fewer Th17 cells than their control counterparts. Plasmacytoid dendritic cells expressing high levels of CD86 and CD40 were elevated in the K/BxNsf synovia.
CONCLUSION
We conclude that Tregs oppose the progression of arthritis by inhibiting the development of RANKL+ cells, homeostatically proliferating CD4+ T cells, Th1, Th2 and mature plasmacytoid dendritic cells, and by inhibiting their influx into joints.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Rheumatoid Fibroblast-like Synoviocytes Downregulate Foxp3 Expression by Regulatory T Cells Via GITRL/GITR Interaction
Sung Hoon Kim, Jeehee Youn
Immune Netw. 2012;12(5):217-221. doi: 10.4110/in.2012.12.5.217.
Reference
-
1. Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002. 2:389–400.
Article2. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003. 299:1057–1061.
Article3. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001. 27:20–21.
Article4. Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005. 54:92–99.
Article5. Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004. 199:971–979.
Article6. Crispin JC, Martínez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003. 21:273–276.
Article7. Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005. 6:345–352.
Article8. Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004. 172:4676–4680.
Article9. Möttönen M, Heikkinen J, Mustonen L, Isomäki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005. 140:360–367.
Article10. Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005. 202:1387–1397.
Article11. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001. 358:903–911.
Article12. Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003. 48:1452–1460.
Article13. Bardos T, Czipri M, Vermes C, Finnegan A, Mikecz K, Zhang J. CD4+CD25+ immunoregulatory T cells may not be involved in controlling autoimmune arthritis. Arthritis Res Ther. 2003. 5:R106–R113.14. van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004. 50:2775–2785.
Article15. Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004. 200:277–285.
Article16. Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004. 200:277–285.
Article17. Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996. 87:811–822.
Article18. Kang SM, Jang E, Paik DJ, Jang YJ, Youn J. CD4+CD25+ regulatory T cells selectively diminish systemic autoreactivity in arthritic K/BxN mice. Mol Cells. 2008. 25:64–69.19. Lee EK, Kang SM, Paik DJ, Kim JM, Youn J. Essential roles of Toll-like receptor-4 signaling in arthritis induced by type II collagen antibody and LPS. Int Immunol. 2005. 17:325–333.
Article20. Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999. 13:2412–2424.
Article21. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005. 6:1123–1132.
Article22. Cao D, Malmström V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003. 33:215–223.
Article23. Green EA, Choi Y, Flavell RA. Pancreatic lymph node-derived CD4(+)CD25(+) Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity. 2002. 16:183–191.24. Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, Nakamura T, Koganei K, Fukushima T, Watanabe M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004. 173:3119–3130.
Article25. Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005. 201:1037–1044.
Article26. Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2008. 105:12439–12444.27. Lande R, Gafa V, Serafini B, Giacomini E, Visconti A, Remoli ME, Severa M, Parmentier M, Ristori G, Salvetti M, Aloisi F, Coccia EM. Plasmacytoid dendritic cells in multiple sclerosis: intracerebral recruitment and impaired maturation in response to interferon-beta. J Neuropathol Exp Neurol. 2008. 67:388–401.28. Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, Jacques S, Ba N, Ittah M, Lepajolec C, Labetoulle M, Ardizzone M, Sibilia J, Fournier C, Chiocchia G, Mariette X. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren's syndrome. Proc Natl Acad Sci U S A. 2006. 103:2770–2775.
Article29. Lande R, Giacomini E, Serafini B, Rosicarelli B, Sebastiani GD, Minisola G, Tarantino U, Riccieri V, Valesini G, Coccia EM. Characterization and recruitment of plasmacytoid dendritic cells in synovial fluid and tissue of patients with chronic inflammatory arthritis. J Immunol. 2004. 173:2815–2824.
Article30. Penna G, Vulcano M, Roncari A, Facchetti F, Sozzani S, Adorini L. Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells. J Immunol. 2002. 169:6673–6676.
Article31. Krug A, Uppaluri R, Facchetti F, Dorner BG, Sheehan KC, Schreiber RD, Cella M, Colonna M. IFN-producing cells respond to CXCR3 ligands in the presence of CXCL12 and secrete inflammatory chemokines upon activation. J Immunol. 2002. 169:6079–6083.
Article32. Vanbervliet B, Bendriss-Vermare N, Massacrier C, Homey B, de Bouteiller O, Brière F, Trinchieri G, Caux C. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J Exp Med. 2003. 198:823–830.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dynamic Frequency of Blood CD4+CD25+ Regulatory T Cells in Rats with Collagen-induced Arthritis
- Peripheral Generation of CD4+ CD25+ Foxp3+ Regulatory T Cells
- Induction of CD4+ Regulatory and Polarized Effector/helper T Cells by Dendritic Cells
- A Study on the Number of Circulating CD4+CD25+Foxp3+ Regulatory T Cells and CD4+CD25-Foxp3+ T Cells in Psoriasis
- Detection of Foreign Antigen-specific CD4+Foxp3+ Regulatory T Cells by MHC Class II Tetramer and Intracellular CD154 Staining