Shellfish and House Dust Mite Allergies: Is the Link Tropomyosin?
- Affiliations
-
- 1Pediatrics, Khoo Teck Puat-National University Children's Medical Institute, National University Health System, Singapore. bee_wah_lee@nuhs.edu.sg
- 2Pediatrics, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
- KMID: 2165925
- DOI: http://doi.org/10.4168/aair.2016.8.2.101
Abstract
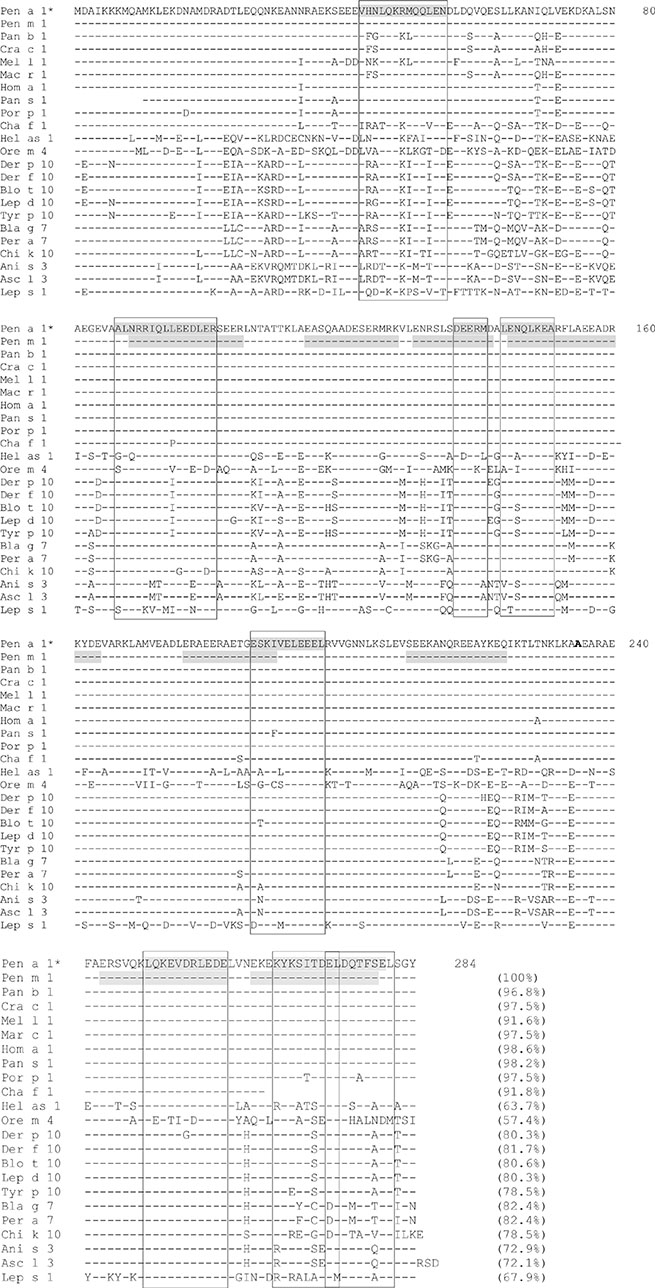
- Crustacean shellfish allergy is an important cause of food allergy and anaphylaxis in Asia. The major allergen in shellfish allergy is tropomyosin, a pan-allergen that is also found in house dust mites and cockroaches. Tropomyosins from house dust mites (HDMs) have a high sequence homology to shellfish tropomyosins, and cross-reactivity between HDM and shrimp tropomyosins has been demonstrated. Exposure to inhaled tropomyosins from house dust mites has been postulated to be the primary sensitizer for shellfish allergy, in a reaction analogous to the oral allergy (inhalant-food) syndrome. This notion is supported by indirect data from the effects of HDM immunotherapy on shellfish allergy, and strong correlations of shellfish and HDM sensitization. HDM immunotherapy has been reported to induce both shrimp allergy in non-allergic patients and shrimp tolerance in shrimp-allergic patients. Epidemiological surveys have also demonstrated a strong correlation between shellfish and HDM sensitization in both hospital-based and community-based studies. Unexposed populations have also been shown to develop sensitization-shellfish sensitization in orthodox Jews with no history of shellfish consumption was associated with HDM sensitization. Reciprocally, HDM sensitization in an Icelandic population living in a HDM-free environment was associated with shrimp sensitization. In vitro IgE inhibition studies on sera in shrimp-allergic Spanish patients indicate that mites are the primary sensitizer in shrimp-allergic patients living in humid and warm climates. Current data supports the hypothesis that tropomyosin is the link between HDM and shellfish allergies. The role of tropomyosin in HDM and shellfish allergies is a fertile field for investigation as it may provide novel immunotherapeutic strategies for shellfish allergy.
Keyword
MeSH Terms
Figure
Cited by 5 articles
-
Spring and allergy
Yoon-Seok Chang
Asia Pac Allergy. 2018;8(2):. doi: 10.5415/apallergy.2018.8.e22.Shellfish/crustacean oral allergy syndrome among national service pre-enlistees in Singapore
Bernard Yu-Hor Thong, Shalini Arulanandam, Sze-Chin Tan, Teck-Choon Tan, Grace Yin-Lai Chan, Justina Wei-Lyn Tan, Mark Chong-Wei Yeow, Chwee-Ying Tang, Jinfeng Hou, Khai-Pang Leong
Asia Pac Allergy. 2018;8(2):. doi: 10.5415/apallergy.2018.8.e18.Allergen Sensitization Pattern by Sex: A Cluster Analysis in Korea
Jungyoon Ohn, Seung Hwan Paik, Eun Jin Doh, Hyun-sun Park, Hyun-Sun Yoon, Soyun Cho
Ann Dermatol. 2017;29(6):735-741. doi: 10.5021/ad.2017.29.6.735.How Different Parts of the World Provide New Insights Into Food Allergy
Elizabeth Huiwen Tham, Donald Y.M. Leung
Allergy Asthma Immunol Res. 2018;10(4):290-299. doi: 10.4168/aair.2018.10.4.290.Detecting Allergens From Black Tiger Shrimp
Penaeus monodon That Can Bind and Cross-link IgE by ELISA, Western Blot, and a Humanized Rat Basophilic Leukemia Reporter Cell Line RS-ATL8
Thanyapat Jarupalee, Pantipa Chatchatee, Kittinan Komolpis, Narissara Suratannon, Sittiruk Roytrakul, Yodying Yingchutrakul, Wanaporn Yimchuen, Patcharavadee Butta, Alain Jacquet, Tanapat Palaga
Allergy Asthma Immunol Res. 2018;10(1):62-76. doi: 10.4168/aair.2018.10.1.62.
Reference
-
1. Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, et al. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010; 126:324–331. 331.e1–331.e7.2. Connett GJ, Gerez I, Cabrera-Morales EA, Yuenyongviwat A, Ngamphaiboon J, Chatchatee P, et al. A population-based study of fish allergy in the Philippines, Singapore and Thailand. Int Arch Allergy Immunol. 2012; 159:384–390.3. Thong BY, Cheng YK, Leong KP, Tang CY, Chng HH. Immediate food hypersensitivity among adults attending a clinical immunology/allergy centre in Singapore. Singapore Med J. 2007; 48:236–240.4. Liew WK, Chiang WC, Goh AE, Lim HH, Chay OM, Chang S, et al. Paediatric anaphylaxis in a Singaporean children cohort: changing food allergy triggers over time. Asia Pac Allergy. 2013; 3:29–34.5. Lertnawapan R, Maek-a-nantawat W. Anaphylaxis and biphasic phase in Thailand: 4-year observation. Allergol Int. 2011; 60:283–289.6. Smit DV, Cameron PA, Rainer TH. Anaphylaxis presentations to an emergency department in Hong Kong: incidence and predictors of biphasic reactions. J Emerg Med. 2005; 28:381–388.7. Hsin YC, Hsin YC, Huang JL, Yeh KW. Clinical features of adult and pediatric anaphylaxis in Taiwan. Asian Pac J Allergy Immunol. 2011; 29:307–312.8. Thalayasingam M, Gerez IF, Yap GC, Llanora GV, Chia IP, Chua L, et al. Clinical and immunochemical profiles of food challenge proven or anaphylactic shrimp allergy in tropical Singapore. Clin Exp Allergy. 2015; 45:687–697.9. Jirapongsananuruk O, Sripramong C, Pacharn P, Udompunturak S, Chinratanapisit S, Piboonpocanun S, et al. Specific allergy to Penaeus monodon (seawater shrimp) or Macrobrachium rosenbergii (freshwater shrimp) in shrimp-allergic children. Clin Exp Allergy. 2008; 38:1038–1047.10. Ayuso R, Lehrer SB, Reese G. Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin). Int Arch Allergy Immunol. 2002; 127:27–37.11. Santos AB, Chapman MD, Aalberse RC, Vailes LD, Ferriani VP, Oliver C, et al. Cockroach allergens and asthma in Brazil: identification of tropomyosin as a major allergen with potential cross-reactivity with mite and shrimp allergens. J Allergy Clin Immunol. 1999; 104:329–337.12. Ayuso R, Reese G, Leong-Kee S, Plante M, Lehrer SB. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol. 2002; 129:38–48.13. Andiappan AK, Puan KJ, Lee B, Nardin A, Poidinger M, Connolly J, et al. Allergic airway diseases in a tropical urban environment are driven by dominant mono-specific sensitization against house dust mites. Allergy. 2014; 69:501–509.14. Gendeh BS, Mujahid SH, Murad S, Rizal M. Atopic sensitization of children with rhinitis in Malaysia. Med J Malaysia. 2004; 59:522–529.15. Chew FT, Lim SH, Goh DY, Lee BW. Sensitization to local dust-mite fauna in Singapore. Allergy. 1999; 54:1150–1159.16. Goh DY, Chew FT, Quek SC, Lee BW. Prevalence and severity of asthma, rhinitis, and eczema in Singapore schoolchildren. Arch Dis Child. 1996; 74:131–135.17. Klaewsongkram J. High prevalence of shellfish and house dust mite allergies in Asia-Pacific: probably not just a coincidence. Asian Pac J Allergy Immunol. 2012; 30:247–248.18. Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010; 104:101–108.19. Breiteneder H, Ebner C. Molecular and biochemical classification of plant-derived food allergens. J Allergy Clin Immunol. 2000; 106:27–36.20. Valenta R, Duchene M, Ebner C, Valent P, Sillaber C, Deviller P, et al. Profilins constitute a novel family of functional plant pan-allergens. J Exp Med. 1992; 175:377–385.21. Ayuso R, Lehrer SB, Reese G. Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin). Int Arch Allergy Immunol. 2002; 127:27–37.22. Reese G, Viebranz J, Leong-Kee SM, Plante M, Lauer I, Randow S, et al. Reduced allergenic potency of VR9-1, a mutant of the major shrimp allergen Pen a 1 (tropomyosin). J Immunol. 2005; 175:8354–8364.23. Zheng LN, Lin H, Pawar R, Li ZX, Li MH. Mapping IgE binding epitopes of major shrimp (Penaeus monodon) allergen with immunoinformatics tools. Food Chem Toxicol. 2011; 49:2954–2960.24. Ayuso R, Reese G, Leong-Kee S, Plante M, Lehrer SB. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol. 2002; 129:38–48.25. Yi FC, Cheong N, Shek LP, Wang DY, Chua KY, Lee BW. Identification of shared and unique immunoglobulin E epitopes of the highly conserved tropomyosins in Blomia tropicalis and Dermatophagoides pteronyssinus. Clin Exp Allergy. 2002; 32:1203–1210.26. van Ree R, Antonicelli L, Akkerdaas JH, Garritani MS, Aalberse RC, Bonifazi F. Possible induction of food allergy during mite immunotherapy. Allergy. 1996; 51:108–113.27. Cortellini G, Spadolini I, Santucci A, Cova V, Conti C, Corvetta A, et al. Improvement of shrimp allergy after sublingual immunotherapy for house dust mites: a case report. Eur Ann Allergy Clin Immunol. 2011; 43:162–164.28. Pevec B, Pevec MR, Markovic AS, Batista I. House dust mite subcutaneous immunotherapy does not induce new sensitization to tropomyosin: does it do the opposite? J Investig Allergol Clin Immunol. 2014; 24:29–34.29. van Ree R, Antonicelli L, Akkerdaas JH, Pajno GB, Barberio G, Corbetta L, et al. Asthma after consumption of snails in house-dustmite-allergic patients: a case of IgE cross-reactivity. Allergy. 1996; 51:387–393.30. Guilloux L, Vuitton DA, Delbourg M, Lagier A, Adessi B, Marchand CR, et al. ross-reactivity between terrestrial snails (Helix species) and house-dust mite (Dermatophagoides pteronyssinus). II. In vitro study. Allergy. 1998; 53:151–158.31. Pajno GB, La Grutta S, Barberio G, Canonica GW, Passalacqua G. Harmful effect of immunotherapy in children with combined snail and mite allergy. J Allergy Clin Immunol. 2002; 109:627–629.32. Asero R. Lack of de novo sensitization to tropomyosin in a group of mite-allergic patients treated by house dust mite-specific immunotherapy. Int Arch Allergy Immunol. 2005; 137:62–65.33. Rossi RE, Monasterolo G, Incorvaia C, Moingeon P, Frati F, Passalacqua G, et al. Lack of neo-sensitization to Pen a 1 in patients treated with mite sublingual immunotherapy. Clin Mol Allergy. 2010; 8:4.34. Chiang WC, Kidon MI, Liew WK, Goh A, Tang JP, Chay OM. The changing face of food hypersensitivity in an Asian community. Clin Exp Allergy. 2007; 37:1055–1061.35. Wang J, Calatroni A, Visness CM, Sampson HA. Correlation of specific IgE to shrimp with cockroach and dust mite exposure and sensitization in an inner-city population. J Allergy Clin Immunol. 2011; 128:834–837.36. Fernandes J, Reshef A, Patton L, Ayuso R, Reese G, Lehrer SB. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy. 2003; 33:956–961.37. Adalsteinsdottir B, Sigurdardottir ST, Gislason T, Kristensen B, Gislason D. What characterizes house dust mite sensitive individuals in a house dust mite free community in Reykjavik, Iceland? Allergol Int. 2007; 56:51–56.38. Gámez C, Zafra M, Boquete M, Sanz V, Mazzeo C, Ibáñez MD, et al. New shrimp IgE-binding proteins involved in mite-seafood cross-reactivity. Mol Nutr Food Res. 2014; 58:1915–1925.39. Boquete M, Iraola V, Morales M, Pinto H, Francisco C, Carballás C, et al. Seafood hypersensitivity in mite sensitized individuals: is tropomyosin the only responsible allergen? Ann Allergy Asthma Immunol. 2011; 106:223–229.40. Yang AC, Arruda LK, Santos AB, Barbosa MC, Chapman MD, Galvão CE, et al. Measurement of IgE antibodies to shrimp tropomyosin is superior to skin prick testing with commercial extract and measurement of IgE to shrimp for predicting clinically relevant allergic reactions after shrimp ingestion. J Allergy Clin Immunol. 2010; 125:872–878.41. Rosmilah M, Shahnaz M, Zailatul HM, Noormalin A, Normilah I. Identification of tropomyosin and arginine kinase as major allergens of Portunus pelagicus (blue swimming crab). Trop Biomed. 2012; 29:467–478.42. Ayuso R, Grishina G, Bardina L, Carrillo T, Blanco C, Ibáñez MD, et al. Myosin light chain is a novel shrimp allergen, Lit v 3. J Allergy Clin Immunol. 2008; 122:795–802.43. Ayuso R, Grishina G, Ibáñez MD, Blanco C, Carrillo T, Bencharitiwong R, et al. Sarcoplasmic calcium-binding protein is an EF-handtype protein identified as a new shrimp allergen. J Allergy Clin Immunol. 2009; 124:114–120.44. Giuffrida MG, Villalta D, Mistrello G, Amato S, Asero R. Shrimp allergy beyond Tropomyosin in Italy: clinical relevance of Arginine Kinase, Sarcoplasmic calcium binding protein and Hemocyanin. Eur Ann Allergy Clin Immunol. 2014; 46:172–177.45. Chen J, Liao Y, Zhang HZ, Zhao H, Chen J, Li HQ. Prevalence of food allergy in children under 2 years of age in three cities in China. Zhonghua Er Ke Za Zhi. 2012; 50:5–9.46. Leung TF, Yung E, Wong YS, Lam CW, Wong GW. Parent-reported adverse food reactions in Hong Kong Chinese pre-schoolers: epidemiology, clinical spectrum and risk factors. Pediatr Allergy Immunol. 2009; 20:339–346.47. Ho MH, Lee SL, Wong WH, Ip P, Lau YL. Prevalence of self-reported food allergy in Hong Kong children and teens--a population survey. Asian Pac J Allergy Immunol. 2012; 30:275–284.48. Wu TC, Tsai TC, Huang CF, Chang FY, Lin CC, Huang IF, et al. Prevalence of food allergy in Taiwan: a questionnaire-based survey. Intern Med J. 2012; 42:1310–1315.49. Santadusit S, Atthapaisalsarudee S, Vichyanond P. Prevalence of adverse food reactions and food allergy among Thai children. J Med Assoc Thai. 2005; 88:Suppl 8. S27–S32.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distribution of House Dust Mites in the Bedroom of Patients with Allergic Rhinitis in Pusan Area
- Avian Mite Dermatitis: Observation of the Causative Mites and Clinical Findings
- Sensitization of house dust mites in the allergic patients and mite ecology in their house dusts
- Patch test and Specific IgE Concentration with House Dust Mite Antigens in Atopic Dermatitis Patients
- House dust mite fauna in western Anatolia, Turkey