Int J Stem Cells.
2016 May;9(1):9-20. 10.15283/ijsc.2016.9.1.9.
Chemicals as the Sole Transformers of Cell Fate
- Affiliations
-
- 1Yazd Cardiovascular Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. bhnmebrahimi@yahoo.com
- KMID: 2164156
- DOI: http://doi.org/10.15283/ijsc.2016.9.1.9
Abstract
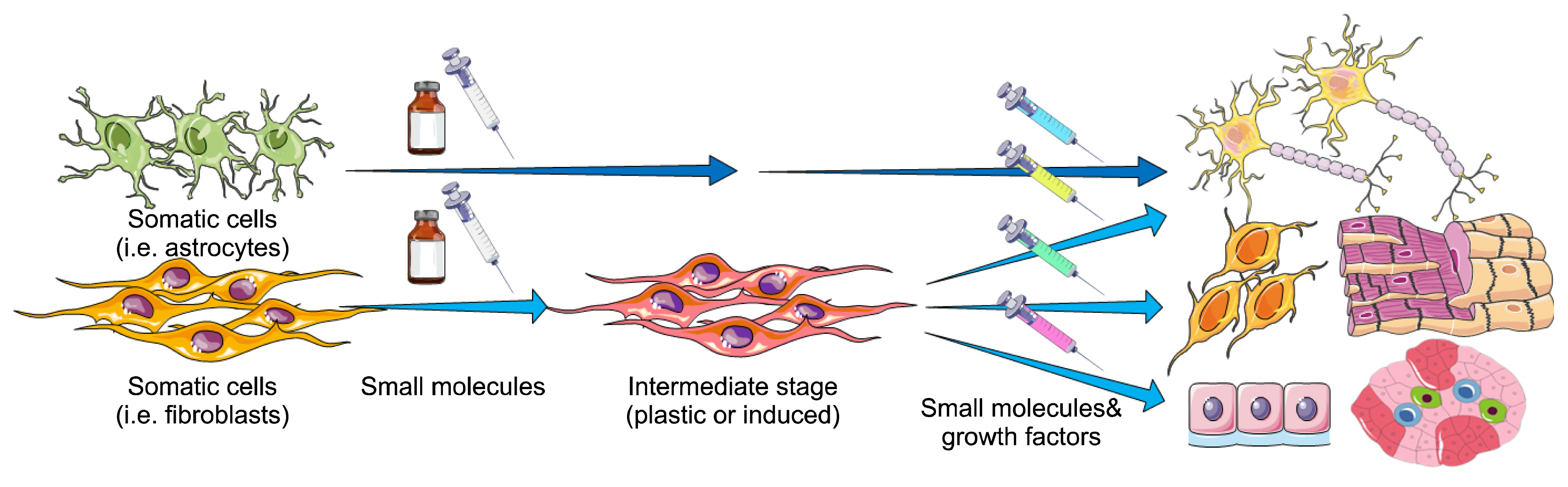
- Forced expression of lineage-specific transcription factors in somatic cells can result in the generation of different cell types in a process named direct reprogramming, bypassing the pluripotent state. However, the introduction of transgenes limits the therapeutic applications of the produced cells. Numerous small-molecules have been introduced in the field of stem cell biology capable of governing self-renewal, reprogramming, transdifferentiation and regeneration. These chemical compounds are versatile tools for cell fate conversion toward desired outcomes. Cell fate conversion using small-molecules alone (chemical reprogramming) has superiority over arduous traditional genetic techniques in several aspects. For instance, rapid, transient, and reversible effects in activation and inhibition of functions of specific proteins are of the profits of small-molecules. They are cost-effective, have a long half-life, diversity on structure and function, and allow for temporal and flexible regulation of signaling pathways. Additionally, their effects could be adjusted by fine-tuning concentrations and combinations of different small-molecules. Therefore, chemicals are powerful tools in cell fate conversion and study of stem cell and chemical biology in vitro and in vivo. Moreover, transgene-free and chemical-only transdifferentiation approaches provide alternative strategies for the generation of various cell types, disease modeling, drug screening, and regenerative medicine. The current review gives an overview of the recent findings concerning transdifferentiation by only small-molecules without the use of transgenes.
MeSH Terms
Figure
Reference
-
References
1. Gurdon JB, Laskey RA, Reeves OR. The developmental capacity of nuclei transplanted from keratinized skin cells of adult frogs. J Embryol Exp Morphol. 1975; 34:93–112. PMID: 1102625.
Article2. Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987; 51:987–1000. DOI: 10.1016/0092-8674(87)90585-X. PMID: 3690668.
Article3. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126:663–676. DOI: 10.1016/j.cell.2006.07.024. PMID: 16904174.
Article4. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131:861–872. DOI: 10.1016/j.cell.2007.11.019. PMID: 18035408.
Article5. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007; 318:1917–1920. DOI: 10.1126/science.1151526. PMID: 18029452.
Article6. Xu J, Du Y, Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell. 2015; 16:119–134. DOI: 10.1016/j.stem.2015.01.013. PMID: 25658369.
Article7. Sancho-Martinez I, Baek SH, Izpisua Belmonte JC. Lineage conversion methodologies meet the reprogramming toolbox. Nat Cell Biol. 2012; 14:892–899. DOI: 10.1038/ncb2567. PMID: 22945254.
Article8. Yu C, Liu K, Tang S, Ding S. Chemical approaches to cell reprogramming. Curr Opin Genet Dev. 2014; 28:50–56. DOI: 10.1016/j.gde.2014.09.006. PMID: 25461450. PMCID: 4747244.
Article9. Jung DW, Kim WH, Williams DR. Reprogram or reboot: small molecule approaches for the production of induced pluripotent stem cells and direct cell reprogramming. ACS Chem Biol. 2014; 9:80–95. DOI: 10.1021/cb400754f.
Article10. Higuchi A, Ling QD, Kumar SS, Munusamy MA, Alarfaj AA, Chang Y, Kao SH, Lin KC, Wang HC, Umezawa A. Generation of pluripotent stem cells without the use of genetic material. Lab Invest. 2015; 95:26–42. DOI: 10.1038/labinvest.2014.132.
Article11. Schlaeger TM, Daheron L, Brickler TR, Entwisle S, Chan K, Cianci A, DeVine A, Ettenger A, Fitzgerald K, Godfrey M, Gupta D, McPherson J, Malwadkar P, Gupta M, Bell B, Doi A, Jung N, Li X, Lynes MS, Brookes E, Cherry AB, Demirbas D, Tsankov AM8, Zon LI, Rubin LL, Feinberg AP, Meissner A, Cowan CA, Daley GQ. A comparison of non-integrating reprogramming methods. Nat Biotechnol. 2015; 33:58–63. DOI: 10.1038/nbt.3070. PMCID: 4329913.
Article12. González F, Boué S, Izpisúa Belmonte JC. Methods for making induced pluripotent stem cells: reprogramming à la carte. Nat Rev Genet. 2011; 12:231–242. DOI: 10.1038/nrg2937.
Article13. Zhou YY, Zeng F. Integration-free methods for generating induced pluripotent stem cells. Genomics Proteomics Bioinformatics. 2013; 11:284–287. DOI: 10.1016/j.gpb.2013.09.008. PMID: 24121200. PMCID: 4357834.
Article14. Goh PA, Caxaria S, Casper C, Rosales C, Warner TT, Coffey PJ, Nathwani AC. A systematic evaluation of integration free reprogramming methods for deriving clinically relevant patient specific induced pluripotent stem (iPS) cells. PLoS One. 2013; 8:e81622. DOI: 10.1371/journal.pone.0081622. PMID: 24303062. PMCID: 3841145.
Article15. Silva M, Daheron L, Hurley H, Bure K, Barker R, Carr AJ, Williams D, Kim HW, French A, Coffey PJ, Cooper-White JJ, Reeve B, Rao M, Snyder EY, Ng KS, Mead BE, Smith JA, Karp JM, Brindley DA, Wall I. Generating iPSCs: translating cell reprogramming science into scalable and robust biomanufacturing strategies. Cell Stem Cell. 2015; 16:13–17. DOI: 10.1016/j.stem.2014.12.013. PMID: 25575079.
Article16. Li W, Jiang K, Ding S. Concise review: A chemical approach to control cell fate and function. Stem Cells. 2012; 30:61–68. DOI: 10.1002/stem.768.
Article17. Li W, Jiang K, Wei W, Shi Y, Ding S. Chemical approaches to studying stem cell biology. Cell Res. 2013; 23:81–91. DOI: 10.1038/cr.2012.182. PMCID: 3541662.
Article18. Li W, Li K, Wei W, Ding S. Chemical approaches to stem cell biology and therapeutics. Cell Stem Cell. 2013; 13:270–283. DOI: 10.1016/j.stem.2013.08.002. PMID: 24012368. PMCID: 3898630.
Article19. Li X, Zuo X, Jing J, Ma Y, Wang J, Liu D, Zhu J, Du X, Xiong L, Du Y, Xu J, Xiao X, Wang J, Chai Z, Zhao Y, Deng H. Small-Molecule-Driven Direct Reprogramming of Mouse Fibroblasts into Functional Neurons. Cell Stem Cell. 2015; 17:195–203. DOI: 10.1016/j.stem.2015.06.003. PMID: 26253201.
Article20. Davies SG, Kennewell PD, Russell AJ, Seden PT, Westwood R, Wynne GM. Stemistry: the control of stem cells in situ using chemistry. J Med Chem. 2015; 58:2863–2894. DOI: 10.1021/jm500838d. PMID: 25590360.
Article21. Cheng L, Hu W, Qiu B, Zhao J, Yu Y, Guan W, Wang M, Yang W, Pei G. Generation of neural progenitor cells by chemical cocktails and hypoxia. Cell Res. 2014; 24:665–679. DOI: 10.1038/cr.2014.32. PMID: 24638034. PMCID: 4042166.
Article22. Hu W, Qiu B, Guan W, Wang Q, Wang M, Li W, Gao L, Shen L, Huang Y, Xie G, Zhao H, Jin Y, Tang B, Yu Y, Zhao J, Pei G. Direct conversion of normal and Alzheimer’s disease human fibroblasts into neuronal cells by small molecules. Cell Stem Cell. 2015; 17:204–212. DOI: 10.1016/j.stem.2015.07.006. PMID: 26253202.
Article23. Pennarossa G, Maffei S, Campagnol M, Tarantini L, Gandolfi F, Brevini TA. Brief demethylation step allows the conversion of adult human skin fibroblasts into insulin-secreting cells. Proc Natl Acad Sci U S A. 2013; 110:8948–8953. DOI: 10.1073/pnas.1220637110. PMID: 23696663. PMCID: 3670366.
Article24. Sayed N, Wong WT, Ospino F, Meng S, Lee J, Jha A, Dexheimer P, Aronow BJ, Cooke JP. Transdifferentiation of human fibroblasts to endothelial cells: role of innate immunity. Circulation. 2015; 131:300–309. DOI: 10.1161/CIRCULATIONAHA.113.007394. PMCID: 4309381.
Article25. Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, Ge J, Xu J, Zhang Q, Zhao Y, Deng H. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013; 341:651–654. DOI: 10.1126/science.1239278. PMID: 23868920.
Article26. Zhang L, Yin JC, Yeh H, Ma NX, Lee G, Chen XA, Wang Y, Lin L, Chen L, Jin P, Wu GY, Chen G. Small molecules efficiently reprogram human astroglial cells into functional neurons. Cell Stem Cell. 2015; 17:735–747. DOI: 10.1016/j.stem.2015.09.012. PMID: 26481520. PMCID: 4675726.
Article27. Fu Y, Huang C, Xu X, Gu H, Ye Y, Jiang C, Qiu Z, Xie X. Direct reprogramming of mouse fibroblasts into cardiomyocytes with chemical cocktails. Cell Res. 2015; 25:1013–1024. DOI: 10.1038/cr.2015.99. PMID: 26292833. PMCID: 4559819.
Article28. Long Y, Wang M, Gu H, Xie X. Bromodeoxyuridine promotes full-chemical induction of mouse pluripotent stem cells. Cell Res. 2015; 25:1171–1174. DOI: 10.1038/cr.2015.96. PMID: 26251165. PMCID: 4650630.
Article29. Zhao Y, Zhao T, Guan J, Zhang X, Fu Y, Ye J, Zhu J, Meng G, Ge J, Yang S, Cheng L, Du Y, Zhao C, Wang T, Su L, Yang W, Deng H. A XEN-like state bridges somatic cells to pluripotency during chemical reprogramming. Cell. 2015; 163:1678–1691. DOI: 10.1016/j.cell.2015.11.017. PMID: 26686652.
Article30. Ye J, Ge J, Zhang X, Cheng L, Zhang Z, He S, Wang Y, Lin H, Yang W, Liu J, Zhao Y, Deng H. Pluripotent stem cells induced from mouse neural stem cells and small intestinal epithelial cells by small molecule compounds. Cell Res. 2016; 26:34–45. DOI: 10.1038/cr.2015.142.
Article31. Cheng L, Gao L, Guan W, Mao J, Hu W, Qiu B, Zhao J, Yu Y, Pei G. Direct conversion of astrocytes into neuronal cells by drug cocktail. Cell Res. 2015; 25:1269–1272. DOI: 10.1038/cr.2015.120. PMID: 26427716. PMCID: 4650423.
Article32. Federation AJ, Bradner JE, Meissner A. The use of small molecules in somatic-cell reprogramming. Trends Cell Biol. 2014; 24:179–187. DOI: 10.1016/j.tcb.2013.09.011. PMCID: 3943685.
Article33. Wijdeven RH, Neefjes J, Ovaa H. How chemistry supports cell biology: the chemical toolbox at your service. Trends Cell Biol. 2014; 24:751–760. DOI: 10.1016/j.tcb.2014.07.002. PMID: 25108565.
Article34. Babos K, Ichida JK. Small molecules take a big step by converting fibroblasts into neurons. Cell Stem Cell. 2015; 17:127–129. DOI: 10.1016/j.stem.2015.07.018. PMID: 26253195.
Article35. Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014; 14:188–202. DOI: 10.1016/j.stem.2013.12.001. PMCID: 3967760.
Article36. Heinrich C, Bergami M, Gascón S, Lepier A, Viganò F, Dimou L, Sutor B, Berninger B, Götz M. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Reports. 2014; 3:1000–1014. DOI: 10.1016/j.stemcr.2014.10.007. PMID: 25458895. PMCID: 4264057.
Article37. Grande A, Sumiyoshi K, López-Juárez A, Howard J, Sakthivel B, Aronow B, Campbell K, Nakafuku M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat Commun. 2013; 4:2373. DOI: 10.1038/ncomms3373. PMID: 23974433. PMCID: 3786770.
Article38. Liu Y, Miao Q, Yuan J, Han S, Zhang P, Li S, Rao Z, Zhao W, Ye Q, Geng J, Zhang X, Cheng L. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. J Neurosci. 2015; 35:9336–9355. DOI: 10.1523/JNEUROSCI.3975-14.2015. PMID: 26109658.
Article39. Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Björklund A, Grealish S, Parmar M. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A. 2013; 110:7038–7043. DOI: 10.1073/pnas.1303829110. PMID: 23530235. PMCID: 3637783.
Article40. Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013; 15:1164–1175. DOI: 10.1038/ncb2843. PMID: 24056302. PMCID: 3867822.
Article41. Vidal SE, Amlani B, Chen T, Tsirigos A, Stadtfeld M. Combinatorial modulation of signaling pathways reveals cell-type-specific requirements for highly efficient and synchronous iPSC reprogramming. Stem Cell Reports. 2014; 3:574–584. DOI: 10.1016/j.stemcr.2014.08.003. PMID: 25358786. PMCID: 4223696.
Article42. Ebrahimi B. Reprogramming barriers and enhancers: strategies to enhance the efficiency and kinetics of induced pluripotency. Cell Regen (Lond). 2015; 4:1–12.
Article43. Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010; 7:150–161. DOI: 10.1016/j.stem.2010.07.007. PMID: 20682444.
Article44. Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010; 142:375–386. DOI: 10.1016/j.cell.2010.07.002. PMID: 20691899. PMCID: 2919844.
Article45. Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports. 2013; 1:235–247. DOI: 10.1016/j.stemcr.2013.07.005. PMID: 24319660. PMCID: 3849259.
Article46. Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011; 13:215–222. DOI: 10.1038/ncb2164. PMID: 21278734.
Article47. Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012; 110:1465–1473. DOI: 10.1161/CIRCRESAHA.112.269035. PMID: 22539765. PMCID: 3380624.
Article48. Wang H, Cao N, Spencer CI, Nie B, Ma T, Xu T, Zhang Y, Wang X, Srivastava D, Ding S. Small molecules enable cardiac reprogramming of mouse fibroblasts with a single factor, Oct4. Cell Rep. 2014; 6:951–960. DOI: 10.1016/j.celrep.2014.01.038. PMID: 24561253. PMCID: 4004339.
Article49. Ebrahimi B. Engineering Cell Fate: The roles of iPSC transcription factors, chemicals, barriers and enhancing factors in reprogramming and transdifferentiation. bioRxiv. 2015; DOI: 10.1101/019455.
Article50. Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012; 151:547–558. DOI: 10.1016/j.cell.2012.09.034. PMID: 23101625. PMCID: 3506423.
Article51. Kurian L, Sancho-Martinez I, Nivet E, Aguirre A, Moon K, Pendaries C, Volle-Challier C, Bono F, Herbert JM, Pulecio J, Xia Y, Li M, Montserrat N, Ruiz S, Dubova I, Rodriguez C, Denli AM, Boscolo FS, Thiagarajan RD, Gage FH, Loring JF, Laurent LC, Izpisua Belmonte JC. Conversion of human fibroblasts to angioblast-like progenitor cells. Nat Methods. 2013; 10:77–83. DOI: 10.1038/nmeth.2255.
Article52. Margariti A, Winkler B, Karamariti E, Zampetaki A, Tsai TN, Baban D, Ragoussis J, Huang Y, Han JD, Zeng L, Hu Y, Xu Q. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci U S A. 2012; 109:13793–13798. DOI: 10.1073/pnas.1205526109. PMID: 22869753. PMCID: 3427074.
Article53. Li J, Huang NF, Zou J, Laurent TJ, Lee JC, Okogbaa J, Cooke JP, Ding S. Conversion of human fibroblasts to functional endothelial cells by defined factors. Arterioscler Thromb Vasc Biol. 2013; 33:1366–1375. DOI: 10.1161/ATVBAHA.112.301167. PMID: 23520160. PMCID: 3898631.
Article54. Ginsberg M, James D, Ding BS, Nolan D, Geng F, Butler JM, Schachterle W, Pulijaal VR, Mathew S, Chasen ST, Xiang J, Rosenwaks Z, Shido K, Elemento O, Rabbany SY, Rafii S. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFβ suppression. Cell. 2012; 151:559–575. DOI: 10.1016/j.cell.2012.09.032. PMID: 23084400. PMCID: 3507451.
Article55. Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013; 19:998–1004. DOI: 10.1038/nm.3267. PMID: 23921754. PMCID: 3967018.
Article56. Xu T, Zhang M, Laurent T, Xie M, Ding S. Concise review: chemical approaches for modulating lineage-specific stem cells and progenitors. Stem Cells Transl Med. 2013; 2:355–361. DOI: 10.5966/sctm.2012-0172. PMID: 23580542. PMCID: 3667559.
Article57. Ebrahimi B. Biological computational approaches: new hopes to improve (re)programming robustness, regenerative medicine and cancer therapeutics. Differentiation. 2016; DOI: 10.1016/j.diff.2016.03.001. PMID: 27056282.
Article58. Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012; 485:593–598. DOI: 10.1038/nature11044. PMID: 22522929. PMCID: 3369107.
Article59. Inagawa K, Miyamoto K, Yamakawa H, Muraoka N, Sadahiro T, Umei T, Wada R, Katsumata Y, Kaneda R, Nakade K, Kurihara C, Obata Y, Miyake K, Fukuda K, Ieda M. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ Res. 2012; 111:1147–1156. DOI: 10.1161/CIRCRESAHA.112.271148. PMID: 22931955.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Granular Cell Tumor on the Sole of a Child
- A Case of Basal Cell Carcinoma of the Sole: Diagnosis Supported by Dermoscopic Features
- Fate and toxicity of spilled chemicals in groundwater and soil environment I: strong acids
- A Study of Skin Thickness in Korean
- Reconstruction of Postburn Scar Contracture of the Sole Using the Medialis Pedis Free Flap