Korean J Radiol.
2015 Oct;16(5):1142-1152. 10.3348/kjr.2015.16.5.1142.
CT Characteristics of Pleural Plaques Related to Occupational or Environmental Asbestos Exposure from South Korean Asbestos Mines
- Affiliations
-
- 1Department of Radiology, School of Medicine, Ewha Womans University, Seoul 07985, Korea. yookkim@ewha.ac.kr
- 2Department of Occupational and Environmental Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- 3Department of Radiology, Dongguk University Ilsan Hospital, Goyang 10326, Korea.
- 4Department of Radiology, Gachon University Gil Medical Center, Incheon 21565, Korea.
- 5Department of Pathology, Yonsei University Wonju College of Medicine, Wonju 26426, Korea.
- KMID: 2160782
- DOI: http://doi.org/10.3348/kjr.2015.16.5.1142
Abstract
OBJECTIVE
This study evaluated the CT characteristics of pleural plaques in asbestos-exposed individuals and compared occupational versus environmental exposure groups.
MATERIALS AND METHODS
This study enrolled 181 subjects with occupational exposure and 98 with environmental exposure from chrysotile asbestos mines, who had pleural plaques confirmed by a chest CT. The CT scans were analyzed for morphological characteristics, the number and distribution of pleural plaques and combined pulmonary fibrosis. Furthermore, the CT findings were compared between the occupational and environmental exposure groups.
RESULTS
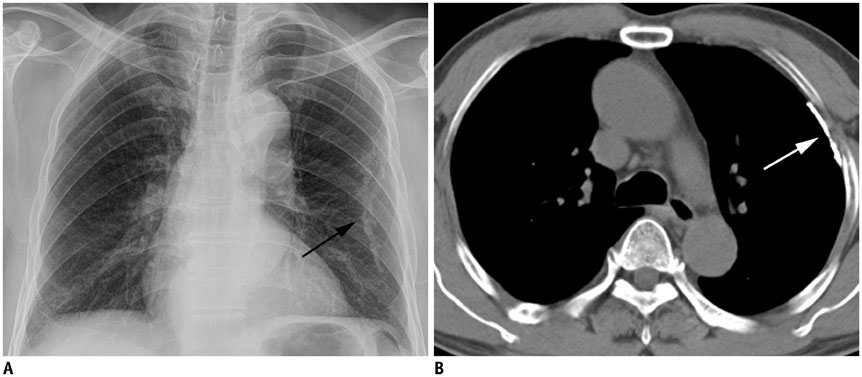
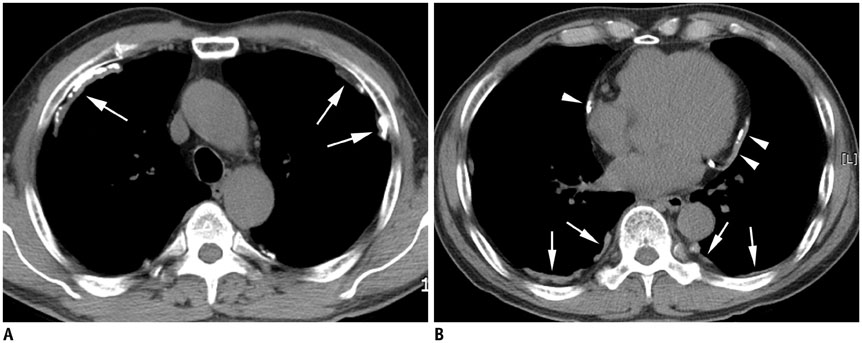
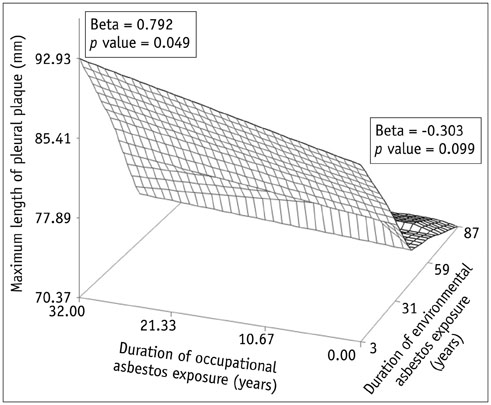
Concerning the 279 subjects, the pleural plaques were single in 2.2% and unilateral in 3.6%, and showed variable widths (range, 1-20 mm; mean, 5.4 +/- 2.7 mm) and lengths (5-310 mm; 72.6 +/- 54.8 mm). The chest wall was the most commonly involved (98.6%), with an upper predominance on the ventral side (upper, 77.8% vs. lower, 55.9%, p < 0.001) and a lower predominance on the dorsal side (upper, 74.9% vs. lower, 91.8%, p = 0.02). Diaphragmatic involvement (78.1%) showed a right-side predominance (right, 73.8% vs. left, 55.6%, p < 0.001), whereas mediastinal plaques (42.7%) were more frequent on the left (right, 17.6% vs. left, 39.4%, p < 0.001). The extent and maximum length of plaques, and presence and severity of combined asbestosis, were significantly higher in the occupational exposure group (p < 0.05).
CONCLUSION
Pleural plaques in asbestos-exposed individuals are variable in number and size; and show a predominant distribution in the upper ventral and lower dorsal chest walls, right diaphragm, and left mediastinum. Asbestos mine workers have a higher extent of plaques and pulmonary fibrosis versus environmentally exposed individuals.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Asbestos, Serpentine/*toxicity
Asbestosis/*etiology/radiography
Asian Continental Ancestry Group
Environmental Pollutants/toxicity
Female
Humans
Male
Middle Aged
Mining
Occupational Exposure
Pleural Diseases/*etiology/radiography
Republic of Korea
Tomography, X-Ray Computed
Asbestos, Serpentine
Environmental Pollutants
Figure
Reference
-
1. McDonald JC, McDonald AD, Armstrong B, Sebastien P. Cohort study of mortality of vermiculite miners exposed to tremolite. Br J Ind Med. 1986; 43:436–444.2. Artvinli M, Bariş YI. Malignant mesotheliomas in a small village in the Anatolian region of Turkey: an epidemiologic study. J Natl Cancer Inst. 1979; 63:17–22.3. Jones RN, McLoud T, Rockoff SD. The radiographic pleural abnormalities in asbestos exposure: relationship to physiologic abnormalities. J Thorac Imaging. 1988; 3:57–66.4. Larson TC, Lewin M, Gottschall EB, Antao VC, Kapil V, Rose CS. Associations between radiographic findings and spirometry in a community exposed to Libby amphibole. Occup Environ Med. 2012; 69:361–366.5. Clin B, Paris C, Ameille J, Brochard P, Conso F, Gislard A, et al. Do asbestos-related pleural plaques on HRCT scans cause restrictive impairment in the absence of pulmonary fibrosis? Thorax. 2011; 66:985–991.6. Cugell DW, Kamp DW. Asbestos and the pleura: a review. Chest. 2004; 125:1103–1117.7. Friedman AC, Fiel SB, Fisher MS, Radecki PD, Lev-Toaff AS, Caroline DF. Asbestos-related pleural disease and asbestosis: a comparison of CT and chest radiography. AJR Am J Roentgenol. 1988; 150:269–275.8. Aberle DR, Gamsu G, Ray CS, Feuerstein IM. Asbestos-related pleural and parenchymal fibrosis: detection with high-resolution CT. Radiology. 1988; 166:729–734.9. Gamsu G, Aberle DR, Lynch D. Computed tomography in the diagnosis of asbestos-related thoracic disease. J Thorac Imaging. 1989; 4:61–67.10. Elshazley M, Shibata E, Hisanaga N, Ichihara G, Ewis AA, Kamijima M, et al. Pleural plaque profiles on the chest radiographs and CT scans of asbestos-exposed Japanese construction workers. Ind Health. 2011; 49:626–633.11. Korosteleva O. Nonparametric regression. In : Korosteleva O, editor. Nonparametric methods in statistics with SAS applications. Florida: CRC Press Taylor & Francis Group;2013. p. 75–96.12. Kim HR. Overview of asbestos issues in Korea. J Korean Med Sci. 2009; 24:363–367.13. Metintas S, Metintas M, Ucgun I, Oner U. Malignant mesothelioma due to environmental exposure to asbestos: follow-up of a Turkish cohort living in a rural area. Chest. 2002; 122:2224–2229.14. Liu XZ, Luo SQ, Wang ZM, Wang MZ, Zhan CL. An investigation of crocidolite contamination and mesothelioma in a rural area of China. Biomed Environ Sci. 1990; 3:156–165.15. Bariş B, Demir AU, Shehu V, Karakoca Y, Kisacik G, Bariş YI. Environmental fibrous zeolite (erionite) exposure and malignant tumors other than mesothelioma. J Environ Pathol Toxicol Oncol. 1996; 15:183–189.16. Peipins LA, Lewin M, Campolucci S, Lybarger JA, Miller A, Middleton D, et al. Radiographic abnormalities and exposure to asbestos-contaminated vermiculite in the community of Libby, Montana, USA. Environ Health Perspect. 2003; 111:1753–1759.17. Metintas M, Metintas S, Hillerdal G, Ucgun I, Erginel S, Alatas F, et al. Nonmalignant pleural lesions due to environmental exposure to asbestos: a field-based, cross-sectional study. Eur Respir J. 2005; 26:875–880.18. Cvitanović S, Znaor L, Konsa T, Ivancević Z, Perić I, Erceg M, et al. Malignant and non-malignant asbestos-related pleural and lung disease: 10-year follow-up study. Croat Med J. 2003; 44:618–625.19. Rohs AM, Lockey JE, Dunning KK, Shukla R, Fan H, Hilbert T, et al. Low-level fiber-induced radiographic changes caused by Libby vermiculite: a 25-year follow-up study. Am J Respir Crit Care Med. 2008; 177:630–637.20. Larson TC, Meyer CA, Kapil V, Gurney JW, Tarver RD, Black CB, et al. Workers with Libby amphibole exposure: retrospective identification and progression of radiographic changes. Radiology. 2010; 255:924–933.21. Churg A, Wright JL, DePaoli L, Wiggs B. Mineralogic correlates of fibrosis in chrysotile miners and millers. Am Rev Respir Dis. 1989; 139:891–896.22. Churg A, Wright J, Wiggs B, Depaoli L. Mineralogic parameters related to amosite asbestos-induced fibrosis in humans. Am Rev Respir Dis. 1990; 142(6 Pt 1):1331–1336.23. Aberle DR, Gamsu G, Ray CS. High-resolution CT of benign asbestos-related diseases: clinical and radiographic correlation. AJR Am J Roentgenol. 1988; 151:883–891.24. Jarad NA, Wilkinson P, Pearson MC, Rudd RM. A new high resolution computed tomography scoring system for pulmonary fibrosis, pleural disease, and emphysema in patients with asbestos related disease. Br J Ind Med. 1992; 49:73–84.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study of Psychosocial Stress of Residents Near Asbestos Mines
- The Prevalence of Asbestos Related Pleural Plaque among Residents Living Near Asbestos Mines in Korea
- Compensation and Diagnosis of Asbestos Related Disease
- Asbestos and environmental diseases
- Review of carcinogenicity of asbestos and proposal of approval standards of an occupational cancer caused by asbestos in Korea