J Rheum Dis.
2014 Apr;21(2):87-90. 10.4078/jrd.2014.21.2.87.
A Case of Condyloma Acuminata in a Virgin Systemic Lupus Erythematosus Patient
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Dong-A University, Busan, Korea. wtchung@dau.ac.kr
- KMID: 2094663
- DOI: http://doi.org/10.4078/jrd.2014.21.2.87
Abstract
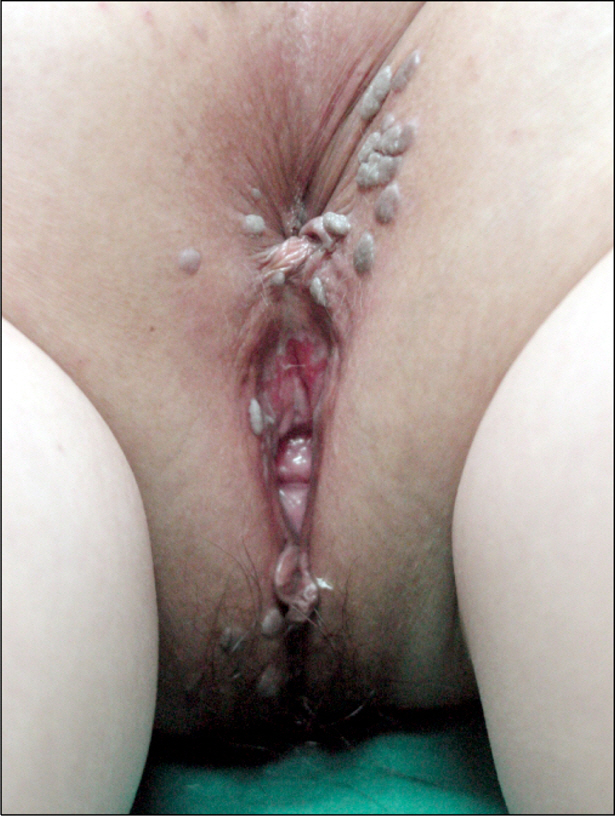
- Anogenital infection with human papillomavirus (HPV) is one of the most common sexually transmitted infections worldwide. The majority of HPV infections are transient and subclinical, with subsequent clearance by the immune system. However, in some individuals, especially those with impaired immunity, HPV infection may persist and result in condyloma acuminatum, pre-cancerous cervical abnormalities, as well as cervical cancer. Because of the intrinsic immunological aberrations and immunosuppressive treatment, patients with systemic lupus erythematosus (SLE) have higher prevalence of anogenital HPV infection, and SLE itself appears to be a major risk factor for HPV infection. HPV infection is sexually transmitted via genital contact; autogenesis of condyloma acuminatum without sexual contact is rare. In this case, a 27-year old virgin female with SLE was admitted to our clinic, presenting anogenital condyloma acuminata. It report that SLE patient can have a disease of anogenital HPV infection, despite the lack of sexual contact. Therefore, we recommend that patients with SLE have regular gynecological evaluations, in addition to prophylactic HPV vaccinations.
MeSH Terms
Figure
Reference
-
1. Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007; 297:813–9.
Article2. Lyrio LD, Grassi MF, Santana IU, Olavarria VG, Gomes Ado N, CostaPinto L, et al. Prevalence of cervical human papillomavirus infection in women with systemic lupus erythematosus. Rheumatol Int. 2013; 33:335–40.
Article3. Forcier M, Musacchio N. An overview of human papillomavirus infection for the dermatologist: disease, diagnosis, management, and prevention. Dermatol Ther. 2010; 23:458–76.
Article4. Lee YH, Choe JY, Park SH, Park YW, Lee SS, Kang YM, et al. Prevalence of human papilloma virus infections and cervical cytological abnormalities among Korean women with systemic lupus erythematosus. J Korean Med Sci. 2010; 25:1431–7.
Article5. Klumb EM, Pinto AC, Jesus GR, Araujo M Jr, Jascone L, Gayer CR, et al. Are women with lupus at higher risk of HPV infection? Lupus. 2010; 19:1485–91.
Article6. Ornstein A, Hatchette T. Human papillomavirus and anogenital warts in children. CMAJ. 2012; 184:321.
Article7. Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003; 157:218–26.
Article8. Nicholls PK, Stanley MA. The immunology of animal papillomaviruses. Vet Immunol Immunopathol. 2000; 73:101–27.
Article9. Zandman-Goddard G, Shoenfeld Y. Infections and SLE. Autoimmunity. 2005; 38:473–85.
Article10. Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007; 356:1928–43.
Article11. Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006; 367:1247–55.
Article12. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an in-terim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007; 369:2161–70.
Article13. Mok CC, Ho LY, Fong LS, To CH. Immunogenicity and safety of a quadrivalent human papillomavirus vaccine in patients with systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 2013; 72:659–64.
Article14. Santana IU, Gomes Ado N, Lyrio LD, Rios Grassi MF, Santiago MB. Systemic lupus erythematosus, human papillomavirus infection, cervical pre-malignant and malignant lesions: a systematic review. Clin Rheumatol. 2011; 30:665–72.
Article15. Febronio MV, Pereira RM, Bonfa E, Takiuti AD, Pereyra EA, Silva CA. Inflammatory cervicovaginal cytology is associated with disease activity in juvenile systemic lupus erythematosus. Lupus. 2007; 16:430–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Penile Carcinoma Transformed from Giant Condyloma Acuminata
- A Case of Penile Cancer Transformed from the Giant Condyloma Acuminata
- A Case of unusual condyloma Acuminatum in an Immunosuppressed Patient
- A Case of Transverse Myelitis as a First Manifestation of Systemic Lupus Erythematosus
- A Ruptured Aneurysm in a Patient with Systemic Lupus Erythematosus: Case Report