J Adv Prosthodont.
2015 Oct;7(5):386-391. 10.4047/jap.2015.7.5.386.
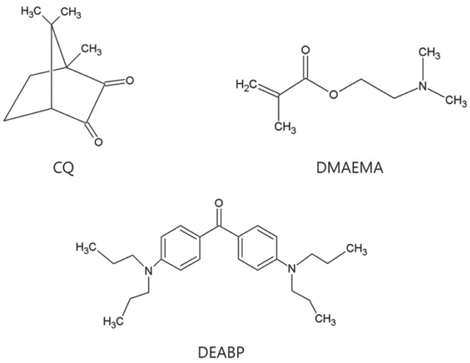
The effect of 4,4'-bis(N,N-diethylamino) benzophenone on the degree of conversion in liquid photopolymer for dental 3D printing
- Affiliations
-
- 1Department of Prosthodontics, School of Dentistry, Kyungpook National University, Daegu, Republic of Korea.
- 2Department of Polymer Science & Engineering, School of Applied Chemical Engineering, Kyungpook National University, Daegu, Republic of Korea.
- 3Department of Dental Biomaterials, School of Dentistry, Kyungpook National University, Daegu, Republic of Korea. tykwon@knu.ac.kr
- KMID: 2083882
- DOI: http://doi.org/10.4047/jap.2015.7.5.386
Abstract
- PURPOSE
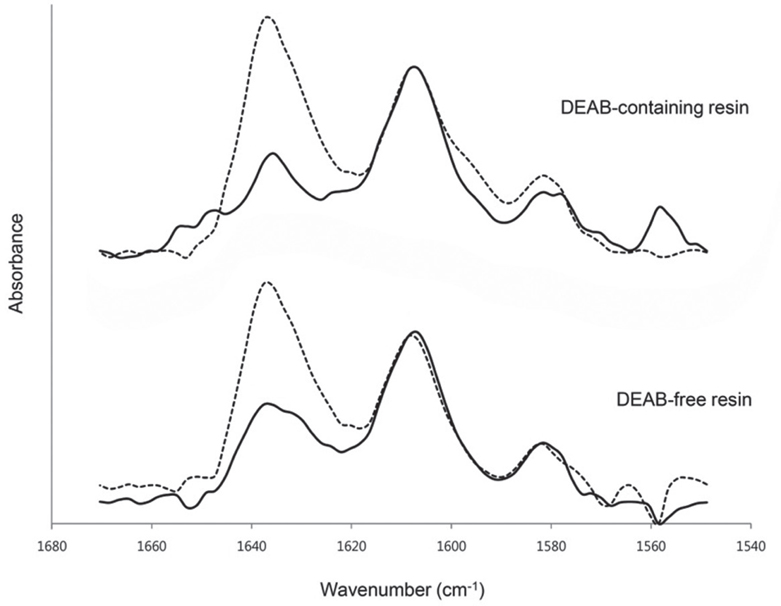
The purpose of this preliminary study was to investigate the effects of adding 4,4'-bis(N,N-diethylamino) benzophenone (DEABP) as a co-initiator to a binary photoinitiating system (camphorquinone-amine) to analyze on the degree of conversion (DC) of a light-cured resin for dental 3D printing.
MATERIALS AND METHODS
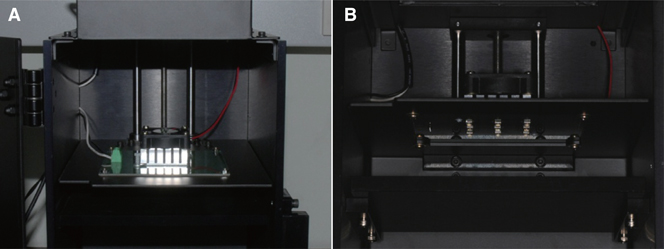
Cylindrical specimens (N=60, n=30 per group, o5 mm x 1 mm) were fabricated using bisphenol A glycerolate dimethacrylate (BisGMA) both with and without DEABP. The freshly mixed resins were exposed to light in a custom-made closed chamber with nine light-emitting diode lamps (wavelength: 405 nm; power: 840 mW/cm2) for polymerization at each incidence of light-irradiation at 10, 30, 60, 180, and 300 seconds, while five specimens at a time were evaluated at each given irradiation point. Fourier-transform infrared (FTIR) spectroscopy was used to measure the DC values of the resins. Two-way analysis of variance and the Duncan post hoc test were used to analyze statistically significant differences between the groups and given times (alpha=.05).
RESULTS
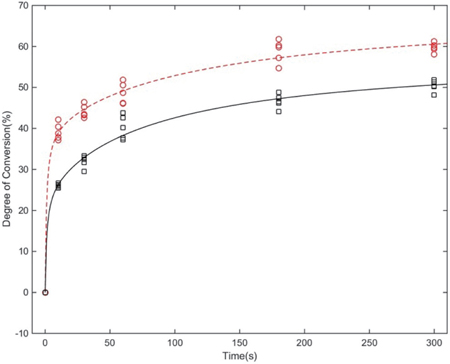
In the DEABP-containing resin, the DC values were significantly higher at all points in time (P<.001), and also the initial polymerization velocity was faster than in the DEABP-free resin.
CONCLUSION
The addition of DEABP significantly enhanced the DC values and, thus, could potentially become an efficient photoinitiator when combined with a camphorquinone-amine system and may be utilized as a more advanced photopolymerization system for dental 3D printing.
Keyword
Figure
Reference
-
1. Bae EJ, Kim JH, Kim WC, Kim HY. Bond and fracture strength of metal-ceramic restorations formed by selective laser sintering. J Adv Prosthodont. 2014; 6:266–271.2. Jardini AL, Larosa MA, Maciel Filho R, Zavaglia CA, Bernardes LF, Lambert CS, Calderoni DR, Kharmandayan P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J Craniomaxillofac Surg. 2014; 42:1877–1884.3. Roper DA, Good BL, McCauley R, Yarlagadda S, Smith J, Good A, Pa A, Mirotznik MS. Additive manufacturing of graded dielectrics. Smart Mater Struct. 2014; 23:045029.4. CW Hull . Apparatus for production of three-dimensional objects by stereolithography. Google Patents. US 45753330 A. 1986.5. Chandramohan D, Marimuthu K. Rapid prototyping/rapid tooling-a over view and its applications in orthopaedics. Int J Adv Eng Tech. 2011; 2:435–448.6. Crivello JV, Reichmanis E. Photopolymer materials and processes for advanced technologies. Chem Mater. 2014; 26:533–548.7. Tonetto MR, Pinto SC, Rastelli Ade N, Borges AH, Saad JR, Pedro FL, de Andrade MF, Bandéca MC. Degree of conversion of polymer-matrix composite assessed by FTIR analysis. J Contemp Dent Pract. 2013; 14:76–79.8. Yoshida K, Greener EH. Effect of photoinitiator on degree of conversion of unfilled light-cured resin. J Dent. 1994; 22:296–299.9. Albuquerque PP, Moreira AD, Moraes RR, Cavalcante LM, Schneider LF. Color stability, conversion, water sorption and solubility of dental composites formulated with different photoinitiator systems. J Dent. 2013; 41:e67–e72.10. Sideridou I, Tserki V, Papanastasiou G. Effect of chemical structure on degree of conversion in light-cured dimethacry-late-based dental resins. Biomaterials. 2002; 23:1819–1829.11. Schneider LF, Pfeifer CS, Consani S, Prahl SA, Ferracane JL. Influence of photoinitiator type on the rate of polymerization, degree of conversion, hardness and yellowing of dental resin composites. Dent Mater. 2008; 24:1169–1177.12. Amintowlieh Y, Tzoganakis C, Hatzikiriakos SG, Penlidis A. Effects of processing variables on polypropylene degradation and long chain branching with UV irradiation. Polym Degrad Stab. 2014; 104:1–10.13. Pongprueksa P, Miletic V, Janssens H, Van Landuyt KL, De Munck J, Godderis L, Van Meerbeek B. Degree of conversion and monomer elution of CQ/amine and TPO adhesives. Dent Mater. 2014; 30:695–701.14. Oliveira DC, Silva CB, Muniz BV, Volpato MC, Costa AR, Sinhoreti MA. Effect of 4-(N,N-dimethylamino)phenethyl alcohol on degree of conversion and cytotoxicity of photo-polymerized CQ-based resin composites. Braz Dent J. 2014; 25:538–542.15. Schroeder WF, Asmussen SL, Cook WD, Vallo CI. Efficiency of 4,4'-bis(N,N-diethylamino) benzophenone for the polymerization of dimethacrylate resins in thick sections. Polym Int. 2011; 60:1362–1369.16. Ely C, Schneider LF, Ogliari FA, Schmitt CC, Corrêa IC, Lima Gda S, Samuel SM, Piva E. Polymerization kinetics and reactivity of alternative initiators systems for use in light-activated dental resins. Dent Mater. 2012; 28:1199–1206.17. Oguri M, Yoshida Y, Yoshihara K, Miyauchi T, Nakamura Y, Shimoda S, Hanabusa M, Momoi Y, Van Meerbeek B. Effects of functional monomers and photo-initiators on the degree of conversion of a dental adhesive. Acta Biomater. 2012; 8:1928–1934.18. Palin WM, Hadis MA, Leprince JG, Leloup G, Boland L, Fleming GJ, Krastl G, Watts DC. Reduced polymerization stress of MAPO-containing resin composites with increased curing speed, degree of conversion and mechanical properties. Dent Mater. 2014; 30:507–516.19. Ikemura K, Ichizawa K, Yoshida M, Ito S, Endo T. UV-VIS spectra and photoinitiation behaviors of acylphosphine oxide and bisacylphosphine oxide derivatives in unfilled, light-cured dental resins. Dent Mater J. 2008; 27:765–774.20. Hammond GS, Wamser CC, Chang CT, Baylor C Jr. Photoreaction of Michler's ketone with benzophenone. Triplet exciplex. J Am Chem Soc. 1970; 92:6362–6363.21. McGinniss VD, Provder T, Kuo C, Gallopo A. Polymerization of methyl methacrylate photoinitiated by 4,4'-bis(N,N-diethylamino) benzophenone.1. Macromolecules. 1978; 11:393–404.22. Santini A, McGuinness N, Nor NA. Degree of conversion of resin-based orthodontic bonding materials cured with single-wave or dual-wave LED light-curing units. J Orthod. 2014; 41:292–298.23. Sahin O, Ozdemir AK, Turgut M, Boztug A, Sumer Z. Investigation of flexural strength and cytotoxicity of acrylic resin copolymers by using different polymerization methods. J Adv Prosthodont. 2015; 7:98–107.24. Urapepon S. Degree of conversion of resin composite cured by light through a translucent fiber posts. J Adv Prosthodont. 2014; 6:194–199.25. Erfan M, Jafarzadeh-Kashi TS, Ghadiri M, Rakhshan V. The effects of dentin bonding agent formulas on their polymerization quality, and together with tooth tissues on their microleakage and shear bond strength: an explorative 3-step experiment. J Adv Prosthodont. 2014; 6:333–345.26. Moon HJ, Shin DH. Effect of CQ-amine ratio on the degree of conversion in resin monomers with binary and ternary photoinitiation systems. Restor Dent Endod. 2012; 37:95–102.27. Moore BK, Platt JA, Borges G, Chu TM, Katsilieri I. Depth of cure of dental resin composites: ISO 4049 depth and microhardness of types of materials and shades. Oper Dent. 2008; 33:408–412.28. Rueggeberg FA, Caughman WF, Curtis JW Jr. Effect of light intensity and exposure duration on cure of resin composite. Oper Dent. 1994; 19:26–32.29. Vitale A, Sangermano M, Bongiovanni R, Burtscher P, Moszner N. Visible Light Curable Restorative Composites for Dental Applications Based on Epoxy Monomer. Materials. 2014; 7:554–562.30. Flury S, Lussi A, Hickel R, Ilie N. Light curing through glass ceramics with a second- and a third-generation LED curing unit: effect of curing mode on the degree of conversion of dual-curing resin cements. Clin Oral Investig. 2013; 17:2127–2137.31. Kim MJ, Kim KH, Kim YK, Kwon TY. Degree of conversion of two dual-cured resin cements light-irradiated through zirconia ceramic disks. J Adv Prosthodont. 2013; 5:464–470.32. Chen Y, Zhou C, Lao J. A layerless additive manufacturing process based on CNC accumulation. Rap Proto J. 2011; 17:218–227.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Three-Dimensional Printing Technology in Orthopedic Surgery
- Mechanical properties and biocompatibility of experimental 3D printing denture base resin
- Medical Applications of 3D Printing and Standardization Issues
- Application of Three-Dimensional Printing in the Fracture Management
- Three-dimensional printing of temporary crowns with polylactic acid polymer using the fused deposition modeling technique: a case series