Lab Anim Res.
2011 Jun;27(2):141-145. 10.5625/lar.2011.27.2.141.
Sensitive and Specific Detection of Mycoplasma species by Consensus Polymerase Chain Reaction and Dot Blot Hybridization
- Affiliations
-
- 1Center for Animal Resources Development, Wonkwang University, Iksan, Republic of Korea. kimoj@wku.ac.kr
- 2Institute of Animal Experiment & Efficacy Evaluation, Wonkwang University, Iksan, Republic of Korea.
- 3Korea DNA Valley Co. Ltd., Iksan, Republic of Korea.
- 4Institute of Biotechnology, Wonkwang University, Iksan, Republic of Korea.
- KMID: 2053663
- DOI: http://doi.org/10.5625/lar.2011.27.2.141
Abstract
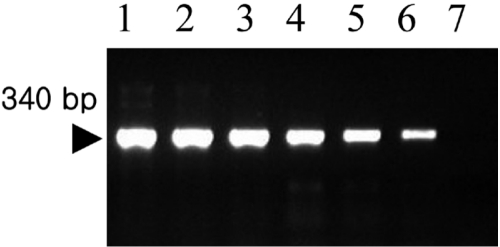
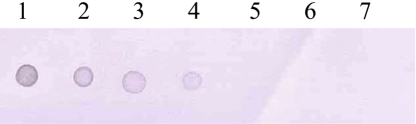
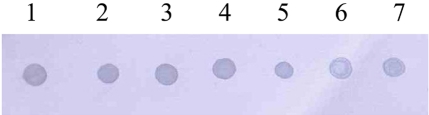
- Mycoplasmas are highly fastidious bacteria, difficult to culture and slow growing. Many species of mycoplasmas are important pathogens that cause respiratory infection in laboratory animals and that are known to affect experimental results obtained with contaminated animals. The aim of the present study was to develop a sensitive and specific assay for the detection of mycoplasma species. To this end, we developed a polymerase chain reaction and dot blot hybridization assay (PCR/DBH) for detecting mycoplasma DNA and evaluated it for its sensitivity and specificity. Mycoplasma consensus primer pairs were used for the amplification of target DNA. When PCR product was visually detected, the limit of detection of the PCR test was 10(2) pg of mycoplasma purified DNA. For DBH, the amplified DNA was labeled by incorporation of digoxigenin (DIG). This DIG-labeled probe was capable of detecting 10(4) pg of purified mycoplasma DNA by DBH. PCR/DBH was more sensitive than PCR or DBH alone and was also very specific. Our PCR/DBH assay can be applied efficiently to confirm the presence of mycoplasma species on clinical samples and to differentiate between mycoplasma species infection and other bacterial infections.
MeSH Terms
Figure
Reference
-
1. McAuliffe L, Ellis RJ, Ayling RD, Nicholas RAJ. Differentiation of Mycoplasma species by 16S ribosomal DNA PCR and denaturing gradient gel electrophoresis fingerprinting. J Clin Microbiol. 2003; 41(10):4844–4847. PMID: 14532239.2. Delgado MO, Timenetsky J. Immunoblot profiles of sera from laboratory rats naturally infected with Mycoplasma pulmonis and technicians exposed to infected animal facilities. Braz J Microbiol. 2001; 32(4):301–304.
Article3. Kim SH, Kim O. Genus-specific polymerase chain reaction to detect mycoplasma species. Lab Anim Res. 2005; 21(3):189–192.4. Muthomi EK, Rurangirwa FR. Passive haemagglutination and complement fixation as diagnostic tests for contagious caprine pleuropneumonia caused by the F-38 strain of mycoplasma. Res Vet Sci. 1983; 35(1):1–4. PMID: 6622834.
Article5. Ball HJ, Finlay D. Diagnostic application of monoclonal antibody (MAb)-based sandwich ELISAs. Methods Mol Biol. 1998; 104:127–132. PMID: 9711648.
Article6. Nicholas RAJ, Santini FG, Clark KM, Palmer NMA, Santis PD, Bashiruddin JB. A comparison of serological tests and gross lung pathology for the detection of contagious bovine pleuropneumonia in two groups of Italian cattle. Vet Rec. 1996; 139(4):89–93. PMID: 8843640.7. Lindsey JR, Baker HJ, Overcash RG, Cassell GH, Hunt CE. Murine chronic respiratory disease. Significance as a research complication and experimental production with Mycoplasma pulmonis. Am J Pathol. 1971; 64(3):675–708. PMID: 4943862.8. Davidson MK, Davis JK, Gambill GP. Whitford HW, editor. Mycoplasmas of laboratory rodents. Mycoplasmosis in Animals: Laboratory Diagnosis. 1994. Ames: Iowa State University Press;p. 97–132.9. Armstrong CH, Yu BH, Yagoda A, Kagnoff MF. Colonization of human by Mycoplasma Canis. J Infect Dis. 1971; 124(6):607–609. PMID: 5166656.10. Yechouron D, Lefebvre J, Robson HG, Rose DL, Tully JG. Fatal septicemia due to Mycoplasma argini: a new human zoonosis. Clin Infect Dis. 1992; 15(3):434–438. PMID: 1520790.11. Kwok S, Higuchi R. Avoiding false positives with PCR. Nature. 1989; 339(6221):237–238. PMID: 2716852.
Article12. Biesiadecka A, Litwinska B. Application of polymerase chain reaction for the detection of herpes simplex virus DNA. Acta Microbiol Pol. 1997; 46(4):405–408. PMID: 9516988.13. Broketa M, Vince A, Drazenovic V, Sim R, Mlinaric-Galinovic G. Non-radioactive digoxigenin DNA labeling and immunologic detection of HSV PCR products. J Clin Virol. 2001; 23(1-2):17–23. PMID: 11595580.
Article14. McNicol AM, Farquharson MA. In situ hybridization and its diagnostic applications in pathology. J Pathol. 1997; 182(3):250–261. PMID: 9349226.15. Duggan MA, Inoue M, McGregor SE, Stuart GC, Morris S, Chang-Poon V, Schepansky A, Honore L. A paired comparison of dot blot hybridization and PCR amplification for HPV testing of cervical scrapes interpreted as CIN 1. Eur J Gynaecol Oncol. 1994; 15(3):178–187. PMID: 7957322.16. Xia JQ, Yason CV, Kibenge FS. Comparison of dot blot hybridization, polymerase chain reaction, and virus isolation for detection of bovine herpesvirus-1 (BHV-1) in artificially infected bovine semen. Can J Vet Res. 1995; 59(2):102–109. PMID: 7648521.17. Hwang SJ, Lee SD, Lu RH, Chan CY, Lai L, Co RL, Tong MJ. Comparison of three different hybridization assays in the quantitative measurement of serum hepatitis B virus DNA. J Virol Methods. 1996; 62(2):123–129. PMID: 9002070.
Article18. Friis NF. The pathogenicity of Mycoplasma flocculare. Acta Vet Scand. 1973; 14(2):344–346. PMID: 4728115.19. Weisburg WG, Tully JG, Rose DL, Petzel JP, Oyalzu H, Yang D, Mandelco L, Sechrest J, Lawrence TG, Van Etten J. A phylogenetic analysis of the mycoplasmas: Basis for their classification. J Bacteriol. 1989; 171(12):6455–6467. PMID: 2592342.20. Kobisch M, Friis NF. Swine mycoplasmoses. Rev Sci Tech. 1996; 15(4):1569–1606. PMID: 9190026.
Article21. Maes D, Verdonck M, Deluyker H, de Kruif A. Enzootic pneumonia in pigs. Vet Quart. 1996; 18(3):104–109.
Article22. Freeman MJ, Armstrong CH, Freeman-Sands LL, Lopez-Osuna M. Serological cross-reactivity of porcine reference antisera to Mycoplasma hyopneumoniae, M. flocculare, M. hyorhinis and M. hyosynoviae indicated by the enzyme-linked immunosorbent assay, complement fixation and indirect hemagglutination tests. Can J Comp Med. 1984; 48(2):202–207. PMID: 6372971.23. Armstrong CH, Freeman MJ, Sands-Freeman L. Crossreactions between Mycoplasma hyopneumoniae and Mycoplasma flocculare: practical implications for the serodiagnosis of mycoplasmal pneumonia of swine. Isr J Med Sci. 1987; 23(6):654–656. PMID: 3667232.24. Cimino GD, Metchette KC, Tessman JW, Hearst JE, Isaaca ST. Post PCR sterilization: a method to control carry-over contamination for the polymerase chain reaction. Nucleic Acids Res. 1991; 19(1):99–107. PMID: 2011516.25. Porter-Jordan K, Rosenberg EI, Keiser WF. Nested polymerase chain reaction assay for the detection of Cytomegalovirus overcomes false positives caused by contamination with fragmented DNA. J Med Virol. 1990; 30(2):85–91. PMID: 2156009.
Article26. Levy R, Najioullah F, Thouvenot D, Bosshard S, Aymard M, Lina B. Evaluation and comparison of PCR and hybridization methods for rapid detection of cytomegalovirus in clinical samples. J Virol Methods. 1996; 62(2):103–111. PMID: 9002068.27. Burns J, Graham AK, Franck C, Fleming KA, Evans MF, McGee JOD. Detection of low copy human papillomavirus DNA and mRNA in routine paraffin sections by non isotopic in situ hybridization. J Clin Pathol. 1987; 40(8):858–864. PMID: 2821078.28. Syrjanen S, Partanen P, Mantvjarvi R, Syrjanen K. Sensitivity of the in situ hybridization techniques using biotin and 35S labelled human papillomavirus (HPV) DNA probes. J Virol Methods. 1988; 19(3-4):225–238. PMID: 2836460.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Sensitive and Specific PCR/dot Blot Hybridization assay for the Detection of Ovine Herpesvirus 2, a Gamma Herpesvirus
- Detection and identification of Mycoplasma species by multiplex PCR in cats
- A sensitive and specific polymerase chain reaction to detect Mycoplasma hyopnemoniae using Mycoplasma protein P97 gene
- Detection of cytomegalovirus DNA in urine culture using polymerase chain reaction
- Study on isolation of Prevotella nigrescens 9336-specific DNA probes using random cloning method