Korean J Gynecol Oncol.
2008 Mar;19(1):9-16. 10.3802/kjgo.2008.19.1.9.
Expression of MTA1 and nm23-H1 protein in ovarian carcinomas in relation to lymph node metastasis
- Affiliations
-
- 1Department of Obstetrics and Gynecology, East-West Neo Medical Center of Kyunghee University, Korea. leeobgy@yahoo.co.kr
- 2Department of Pathology, Kyunghee University College of Medicine, Seoul, Korea.
- KMID: 1979497
- DOI: http://doi.org/10.3802/kjgo.2008.19.1.9
Abstract
-
OBJECTIVE
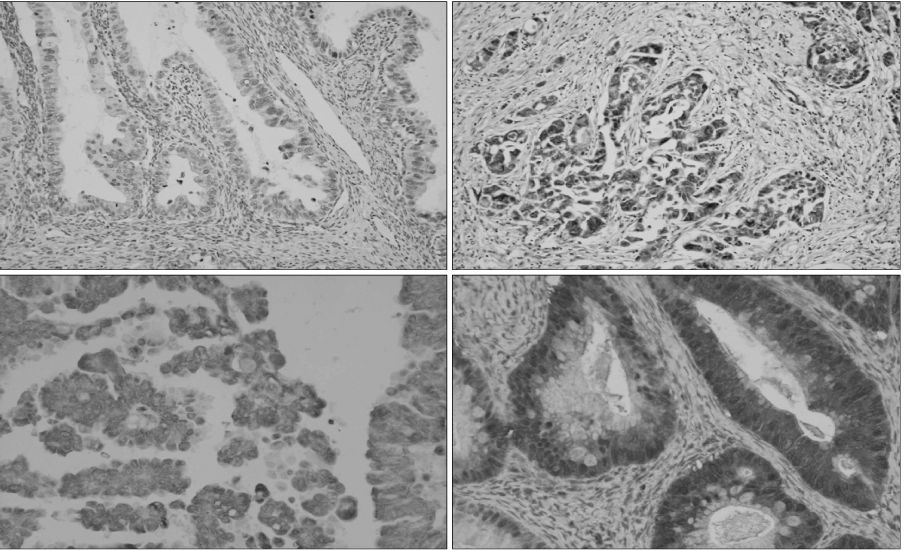
Cancer metastasis is a complex process involving a sequential series of multi-step genetic events, which produces an imbalance between stimulatory and inhibitory genes for metastasis. Presently, we examined the expression of metastatic tumor antigen 1 (MTA1) and nonmetastatic protein 23 homologue H1 (nm23-H1) proteins in metastasized epithelial ovarian cancer cells.
METHODS
Fifty-one primary epithelial ovarian tumors and corresponding lymph nodes (LNs) were examined immunohistochemically for expression of MTA1 and nm23-H1. Expression of these proteins was statistically evaluated.
RESULTS
The frequency of MTA1 expression was 30.3% (10/33) in stage III/IV LNs but was absent (0/18) in stage I/II LNs (p=0.01). MTA1 expression was observed in 50% (6/12) of metastasizing LNs but in only 10.3% (4/39) of non-metastasizing LNs (p=0.01). In contrast with MTA1, nm23-H1 expression was evident in 16 of 18 (88.9%) stage I/II ovarian cancer tissue samples but only in 20 of 33 (60.6%) stage III/IV tissues (p=0.05), and nm23-H1 production was also observed in 75.6% (34/45) of ovarian cancer tissue with residual tumors under 2 cm in diameter, but in 2/6 (33.3%) of cancer tissue with residual tumors exceeding 2 cm in diameter (p=0.03).
CONCLUSION
The degree of expression and imbalance of MTA1 and nm23H1 are correlated with ovarian cancer LN metastasis.
Keyword
MeSH Terms
Figure
Reference
-
1. Annual report of gynecologic cancer registry program in Korea for 2004 (Jan. 1st, 2004-Dec, 31st, 2004). Korean J Obstet Gynecol. 2007. 50:28–78.2. Fidler IJ. Critical factors in the biology of human cancer metastasis: Twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990. 50:6130–6138.
Article3. Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: An imbalance of positive and negative regulation. Cell. 1991. 64:327–336.
Article4. Pencil SD, Toh Y, Nicolson GL. Candidate metastasis-associated genes of the rat 13762NF mammary adenocarcinoma. Breast Cancer Res Treat. 1993. 25:165–174.5. Toh Y, Pencil SD, Nicolson GL. A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. J Biol Chem. 1994. 269:22958–22963.6. Toh Y, Pencil SD, Nicolson GL. Analysis of the complete sequence of the novel metastasis-associated candidate gene, mta1, differentially expressed in mammary adenocarcinoma and breast cancer cell lines. Gene. 1995. 159:97–104.
Article7. Toh Y, Oki E, Oda S, Tokunaga E, Ohno S, Maehara Y, et al. Overexpression of the MTA1 gene in gastrointestinal carcinomas: Correlation with invasion and metastasis. Int J Cancer. 1997. 74:459–463.
Article8. Toh Y, Kuwano H, Mori M, Nicolson GL, Sugimachi K. Overexpression of metastasis-associated MTA1 mRNA in invasive oesophageal carcinomas. Br J Cancer. 1999. 79:1723–1726.
Article9. Steeg PS, Bevilacqua G, Kopper L, Thorgeirsson UP, Talmadge JE, Liotta LA, et al. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst. 1988. 80:200–204.
Article10. Leone A, Flatow U, VanHoutte K, Steeg PS. Transfection of human nm23-H1 into the human MDA-MB-435 breast carcinoma cell line: Effects on tumor metastatic potential, colonization and enzymatic activity. Oncogene. 1993. 8:2325–2333.11. de la Rosa A, Williams RL, Steeg PS. Nm23/nucleoside diphosphate kinase: Toward a structural and biochemical understanding of its biological functions. Bioessays. 1995. 17:53–62.12. Leone A, Seeger RC, Hong CM, Hu YY, Arboleda MJ, Brodeur GM, et al. Evidence for nm23 RNA overexpression, DNA amplification and mutation in aggressive childhood neuroblastomas. Oncogene. 1993. 8:855–865.13. Srivatsa PJ, Cliby WA, Keeney GL, Dodson MK, Suman VJ, Roche PC, et al. Elevated nm23 protein expression is correlated with diminished progressionfree survival in patients with epithelial ovarian carcinoma. Gynecol Oncol. 1996. 60:363–372.14. Leary JA, Kerr J, Chenevix-Trench G, Doris CP, Hurst T, Houghton CR, et al. Increased expression of the NME1 gene is associated with metastasis in epithelial ovarian cancer. Int J Cancer. 1995. 64:189–195.15. Ferrandina G, Scambia G, Marone M, Benedetti Panici P, Giannitelli C, Pernisco S, et al. nm23 in ovarian cancer. Correlation with clinicopathological and biochemical parameters. Ann N Y Acad Sci. 1996. 784:509–512.16. Iguchi H, Imura G, Toh Y, Ogata Y. Expression of MTA1, a metastasis-associated gene with histone deacetylase activity in pancreatic cancer. Int J Oncol. 2000. 16:1211–1241.
Article17. Sasaki H, Moriyama S, Nakashima Y, Kobayashi Y, Yukiue H, Kaji M, et al. Expression of the MTA1 mRNA in advanced lung cacner. Lung Cancer. 2002. 35:149–154.
Article18. Sasaki H, Yukiue H, Kobayashi Y, Nakashima Y, Kaji M, Fukai I, et al. Expression of the MTA1 mRNA in thymoma patients. Cancer Lett. 2001. 174:159–163.19. Mahoney MG, Simpsom A, Jost M, Noe M, Kari C, Pepe D, et al. Metastasis-associated protein (MTA1) enhances migration, invasion, and anchorageindependent survival of immortalized human keratinocytes. Oncogene. 2002. 21:2161–2170.20. Yoon MS, Jang SK, Lee DH, Kim KH, Na YJ, Kim JY, et al. MTA1 expression in epithelial ovarian neoplasm. Korean J Obstet Gynecol. 2006. 49:1463–1470.
Article21. Varesco L, Caligo MA, Simi P, Black DM, Nardini V, Casarino L, et al. The NM23 gene maps to human chromosome band 17q22 and shows a restriction fragment length polymorphism with BglII. Genes Chromosomes Cancer. 1992. 4:84–88.
Article22. Lacomb ML, Milon L, Munier A, Mehus JG, Lambeth DO. The human nm23/nucleoside diphosphate kinases. J Bioenerg Biomembr. 2000. 32:247–258.23. Zhu J, Tseng YH, Kantor JD, Rhodes CJ, Zetter BR, Moyers JS, et al. Interaction of the Ras-related protein associated with diabetes rad and the putative tumor metastasis suppressor NM23 provides a novel mechanism of GTPase regulation. Proc Natl Acad Sci USA. 1999. 96:14911–14918.24. Lombardi D, Sacchi A, D'Angostino G, Tibursi G. The association of the Nm23-H1 protein and beta-tubulin correlates with cell differentiation. Exp Cell Res. 1995. 217:267–271.25. Caligo MA, Cipollini G, Fiore L, Calvo S, Basolo F, Collecchi P, et al. NM23 gene expression correlates with cell growth rate and S-phase. Int J Cancer. 1995. 60:837–842.26. Leone A, Flatow U, Van Houtte K, Steeg PS. Transfection of human nm23H1 into human MDAMB-435 breast carcinoma cell line: Effects on tumour metastatic potential, colonization and enzymatic activity. Oncogene. 1993. 8:2325–2333.27. Hwang SH, Oh SH, Choi YK. The clinical significance of mutation in the p53, DCC and nm23 genes in patients with gastric carcinoma. Korean J Gastroenterol. 2001. 38:325–335.28. Bae JW, Kim J, Cho MY, Lee ES, Koo BH. Nm23 protein as a prognostic factor in lymph node negative breast cancers. J Korean Surg Soc. 1999. 57:836–842.29. Scambia G, Ferrandina G, Marone M, Benedetti Panici P, Giannitelli C, Piantelli M, et al. Nm23 in ovarian cancer: Correlation with clinical outcome and other clinico-pathological and biochemical prognostic parameters. J Clin Oncol. 1996. 14:334–342.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- nm23 Protein Immunohistochemical Expression in Colorectal Carcinoma and its Relationship with Other Prognostic Factors
- Immunohistochemical Study of p53 and nm23-H1 Protein in Gastric Carcinoma
- Expression of p53 and nm23 Proteins in Non-Small Cell Lung Cancer
- The clinical relevance of nm23 protein expression in resected gastric cancer patient
- Expression of p53 and NDP-K/nm23 in gastric carcinomas: association with metastasis and clinicopathologic parameters