J Periodontal Implant Sci.
2010 Dec;40(6):283-288. 10.5051/jpis.2010.40.6.283.
Incomplete bone formation after sinus augmentation: A case report on radiological findings by computerized tomography at follow-up
- Affiliations
-
- 1Department of Periodontology, Kyung Hee University School of Dentistry, Seoul, Korea. chungjh@khu.ac.kr
- 2Institute of Oral Biology, Kyung Hee University School of Dentistry, Seoul, Korea.
- KMID: 1783573
- DOI: http://doi.org/10.5051/jpis.2010.40.6.283
Abstract
- PURPOSE
The aim of this case report is to present a case of incomplete bone formation after sinus augmentation.
METHODS
A patient having alveolar bone resorption of the maxillary posterior edentulous region and advanced pneumatization of the maxillary sinus was treated with sinus elevation using deproteinized bovine bone in the Department of Periodontology, Kyung Hee University School of Dentistry and re-evaluated with computed tomography (CT) follow-up.
RESULTS
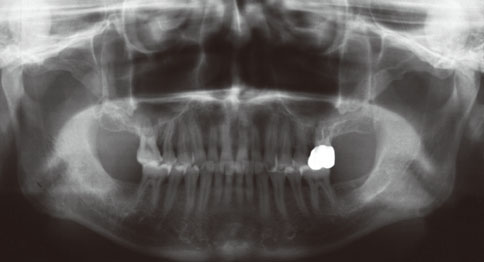
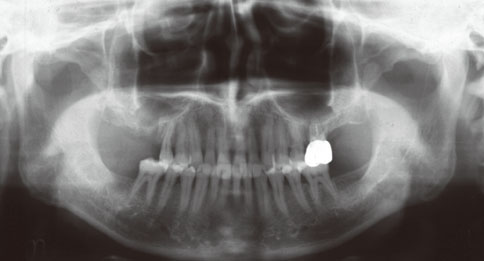
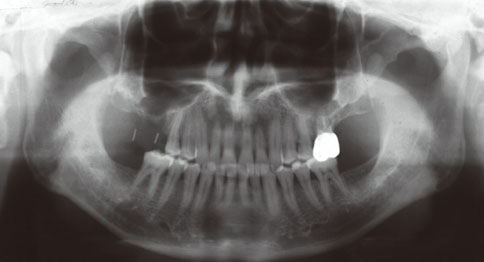
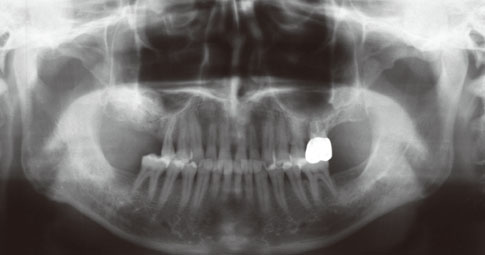
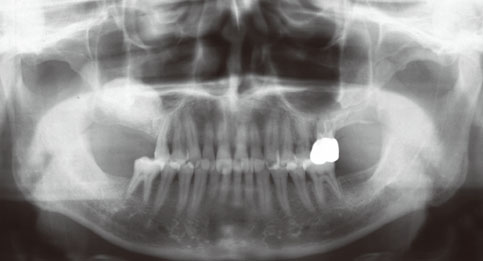
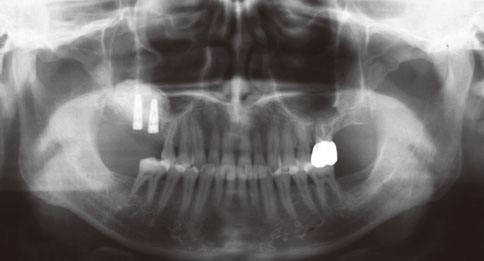
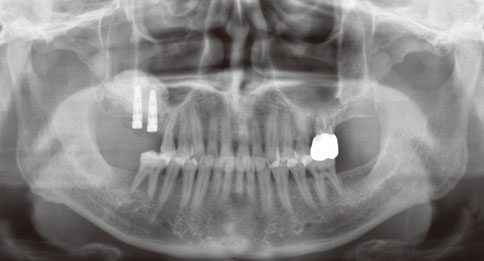
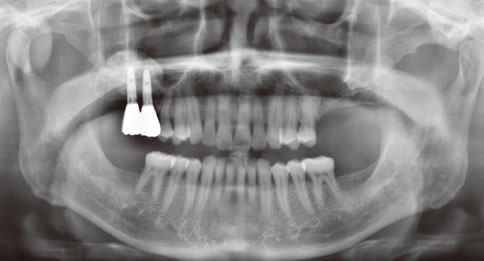
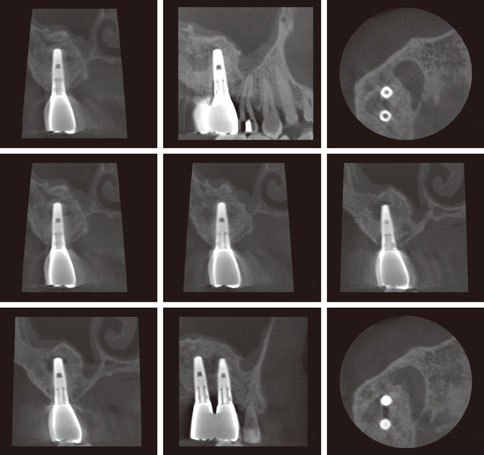
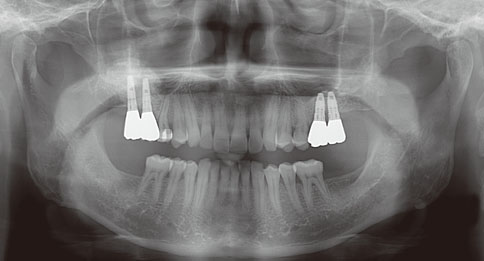
Even though there were no significant findings or abnormal radiolucency on the panoramic radiograph, incomplete bone formation in the central portion of the augmented sinus was found fortuitously in the CT scan. The CT scan revealed peri-implant radiolucency in the apical portion of the implant placed in the augmented maxillary sinus. Nevertheless, the dental implants placed in the grafted sinus still functioned well at over 15 months follow-up.
CONCLUSIONS
The result of this case suggests that patients who received maxillary sinus augmentation may experience incomplete bone formation. It is possible that 1) osteoconductive graft material with poor osteogenic potential, 2) overpacking of graft material that restricts the blood supply, and 3) bone microbial contamination may cause the appearance of incomplete bone formation after sinus augmentation. Further studies are needed to elucidate the mechanism of this unexpected result and care must be taken to prevent it.
Keyword
MeSH Terms
Figure
Reference
-
1. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980. 38:613–616.2. Peleg M, Chaushu G, Mazor Z, Ardekian L, Bakoon M. Radiological findings of the post-sinus lift maxillary sinus: a computerized tomography follow-up. J Periodontol. 1999. 70:1564–1573.
Article3. Murakami K, Itoh T, Watanabe S, Naito T, Yokota M. Periodontal and computer tomography scanning evaluation of endosseous implants in conjunction with sinus lift procedure. A 6-case series. J Periodontol. 1999. 70:1254–1259.
Article4. Pjetursson BE, Tan WC, Zwahlen M, Lang NP. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J Clin Periodontol. 2008. 35:216–240.
Article5. Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann Periodontol. 2003. 8:328–343.
Article6. Mardinger O, Nissan J, Chaushu G. Sinus floor augmentation with simultaneous implant placement in the severely atrophic maxilla: technical problems and complications. J Periodontol. 2007. 78:1872–1877.
Article7. Barone A, Santini S, Sbordone L, Crespi R, Covani U. A clinical study of the outcomes and complications associated with maxillary sinus augmentation. Int J Oral Maxillofac Implants. 2006. 21:81–85.8. Schwartz-Arad D, Herzberg R, Dolev E. The prevalence of surgical complications of the sinus graft procedure and their impact on implant survival. J Periodontol. 2004. 75:511–516.
Article9. Zijderveld SA, van den Bergh JP, Schulten EA, ten Bruggenkate CM. Anatomical and surgical findings and complications in 100 consecutive maxillary sinus floor elevation procedures. J Oral Maxillofac Surg. 2008. 66:1426–1438.
Article10. Hallman M, Sennerby L, Zetterqvist L, Lundgren S. A 3-year prospective follow-up study of implant-supported fixed prostheses in patients subjected to maxillary sinus floor augmentation with a 80:20 mixture of deproteinized bovine bone and autogenous bone clinical, radiographic and resonance frequency analysis. Int J Oral Maxillofac Surg. 2005. 34:273–280.
Article11. Hallman M, Cederlund A, Lindskog S, Lundgren S, Sennerby L. A clinical histologic study of bovine hydroxyapatite in combination with autogenous bone and fibrin glue for maxillary sinus floor augmentation: results after 6 to 8 months of healing. Clin Oral Implants Res. 2001. 12:135–143.
Article12. Skoglund A, Hising P, Young C. A clinical and histologic examination in humans of the osseous response to implanted natural bone mineral. Int J Oral Maxillofac Implants. 1997. 12:194–199.13. Yildirim M, Spiekermann H, Biesterfeld S, Edelhoff D. Maxillary sinus augmentation using xenogenic bone substitute material Bio-Oss in combination with venous blood: a histologic and histomorphometric study in humans. Clin Oral Implants Res. 2000. 11:217–229.
Article14. Fuerst G, Tangl S, Gruber R, Gahleitner A, Sanroman F, Watzek G. Bone formation following sinus grafting with autogenous bone-derived cells and bovine bone mineral in minipigs: preliminary findings. Clin Oral Implants Res. 2004. 15:733–740.
Article15. Roldan JC, Jepsen S, Schmidt C, Knuppel H, Rueger DC, Acil Y, et al. Sinus floor augmentation with simultaneous placement of dental implants in the presence of platelet-rich plasma or recombinant human bone morphogenetic protein-7. Clin Oral Implants Res. 2004. 15:716–723.
Article16. Busenlechner D, Huber CD, Vasak C, Dobsak A, Gruber R, Watzek G. Sinus augmentation analysis revised: the gradient of graft consolidation. Clin Oral Implants Res. 2009. 20:1078–1083.
Article17. Quinones CR, Hurzeler MB, Schupbach P, Kirsch A, Blum P, Caffesse RG, et al. Maxillary sinus augmentation using different grafting materials and osseointegrated dental implants in monkeys. Part II. Evaluation of porous hydroxyapatite as a grafting material. Clin Oral Implants Res. 1997. 8:487–496.
Article18. Haas R, Donath K, Fodinger M, Watzek G. Bovine hydroxyapatite for maxillary sinus grafting: comparative histomorphometric findings in sheep. Clin Oral Implants Res. 1998. 9:107–116.
Article19. Margolin MD, Cogan AG, Taylor M, Buck D, McAllister TN, Toth C, et al. Maxillary sinus augmentation in the non-human primate: a comparative radiographic and histologic study between recombinant human osteogenic protein-1 and natural bone mineral. J Periodontol. 1998. 69:911–919.
Article20. Hurzeler MB, Quinones CR, Kirsch A, Gloker C, Schupbach P, Strub JR, et al. Maxillary sinus augmentation using different grafting materials and dental implants in monkeys. Part I. Evaluation of anorganic bovine-derived bone matrix. Clin Oral Implants Res. 1997. 8:476–486.21. Hurzeler MB, Quinones CR, Kirsch A, Schupbach P, Krausse A, Strub JR, et al. Maxillary sinus augmentation using different grafting materials and dental implants in monkeys. Part III. Evaluation of autogenous bone combined with porous hydroxyapatite. Clin Oral Implants Res. 1997. 8:401–411.22. Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998. 16:155–162.
Article23. Schliephake H, Knebel JW, Aufderheide M, Tauscher M. Use of cultivated osteoprogenitor cells to increase bone formation in segmental mandibular defects: an experimental pilot study in sheep. Int J Oral Maxillofac Surg. 2001. 30:531–537.
Article24. Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000. 49:328–337.
Article25. De Kok IJ, Peter SJ, Archambault M, van den Bos C, Kadiyala S, Aukhil I, et al. Investigation of allogeneic mesenchymal stem cell-based alveolar bone formation: preliminary findings. Clin Oral Implants Res. 2003. 14:481–489.
Article26. Rosenberg MM. Free osseous tissue autografts as a predictable procedure. J Periodontol. 1971. 42:195–209.
Article27. Garrett S, Loos B, Chamberlain D, Egelberg J. Treatment of intraosseous periodontal defects with a combined adjunctive therapy of citric acid conditioning, bone grafting, and placement of collagenous membranes. J Clin Periodontol. 1988. 15:383–389.
Article28. Vastardis S, Yukna RA, Mayer ET, Atkinson BL. Periodontal regeneration with peptide-enhanced anorganic bone matrix in particulate and putty form in dogs. J Periodontol. 2005. 76:1690–1696.
Article29. Verdugo F, Castillo A, Moragues MD, Ponton J. Bone microbial contamination influences autogenous grafting in sinus augmentation. J Periodontol. 2009. 80:1355–1364.
Article30. Choukroun J, Simonpieri A, Del Corso M, Mazor Z, Sammartino G, Dohan Ehrenfest DM. Controlling systematic perioperative anaerobic contamination during sinus-lift procedures by using metronidazole: an innovative approach. Implant Dent. 2008. 17:257–270.
Article31. Hanisch O, Lozada JL, Holmes RE, Calhoun CJ, Kan JY, Spiekermann H. Maxillary sinus augmentation prior to placement of endosseous implants: a histomorphometric analysis. Int J Oral Maxillofac Implants. 1999. 14:329–336.32. Nkenke E, Stelzle F. Clinical outcomes of sinus floor augmentation for implant placement using autogenous bone or bone substitutes: a systematic review. Clin Oral Implants Res. 2009. 20:Suppl 4. 124–133.
Article33. Raghoebar GM, Timmenga NM, Reintsema H, Stegenga B, Vissink A. Maxillary bone grafting for insertion of endosseous implants: results after 12-124 months. Clin Oral Implants Res. 2001. 12:279–286.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Maxillary sinus floor augmentation with anorganic bovine bone: Histologic evaluation in humans
- Maxillary sinus bone graft using particulated ramal autobone and bovine bone
- Management of Perioperative Pathologic Conditions Involving Maxillary Sinus for Dental Implant Placement and Sinus Augmentation: Report of Case Series and Literature Review
- Maxillary Sinus Augmentation Using Macroporous Biphasic Calcium Phosphate (MBCP(TM)): Three Case Report With Histologic Evaluation
- Combined Sinus Floor and Alveolar Ridge Augmentation Simultaneously Performed with Extraction of Ankylosed Maxillary Molar: A Case Report