J Korean Surg Soc.
2009 Nov;77(5):344-352. 10.4174/jkss.2009.77.5.344.
The Expression of Matrix Metalloproteinase according to Hydrostatic Pressure in Varicose Veins
- Affiliations
-
- 1Division of Transplantation and Vascular Surgery, Department of Surgery, Kyungpook National University School of Medicine, Daegu, Korea. shuh@knu.ac.kr
- KMID: 1750704
- DOI: http://doi.org/10.4174/jkss.2009.77.5.344
Abstract
- PURPOSE
The expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinase (TIMPs) caused by hydrostatic pressure stress is important in the development of varicose veins (VVs). This study was performed to analyse the expression of various MMPs and TIMPs according to the hydrostatic stress and the anatomical level of human great saphenous vein (GSV).
METHODS
Forty-nine vein samples were obtained from 10 patients with VVs (control group), and 34 samples from 7 VV patients after 1-hour hydrostatic stress just before surgery (stress group) at each anatomical site (proximal, Hunter, Dodd, and Boyd perforators) of GSV. Light microscopic examination and immunohistochemistry for MMP-1, -2, -9, -13 and TIMP-1, -2 were performed.
RESULTS
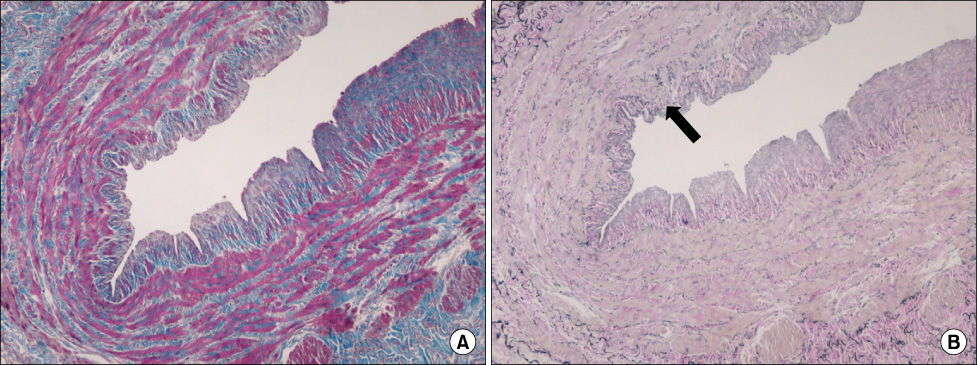
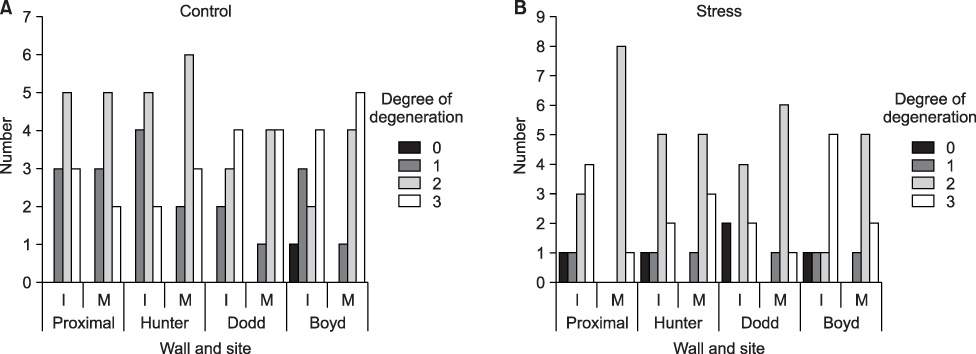
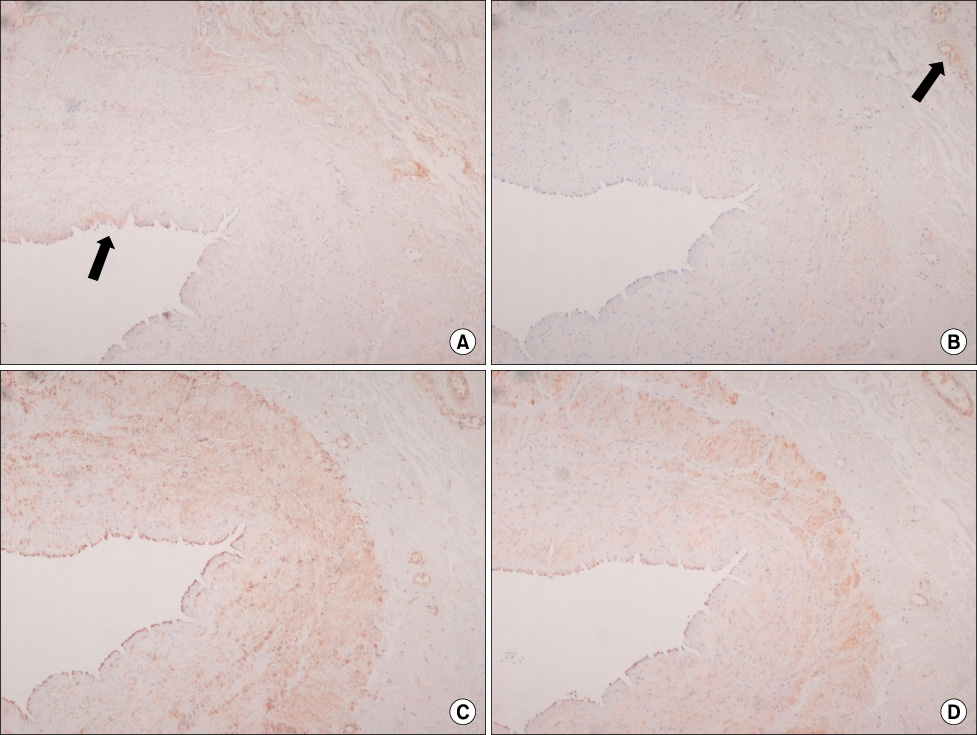
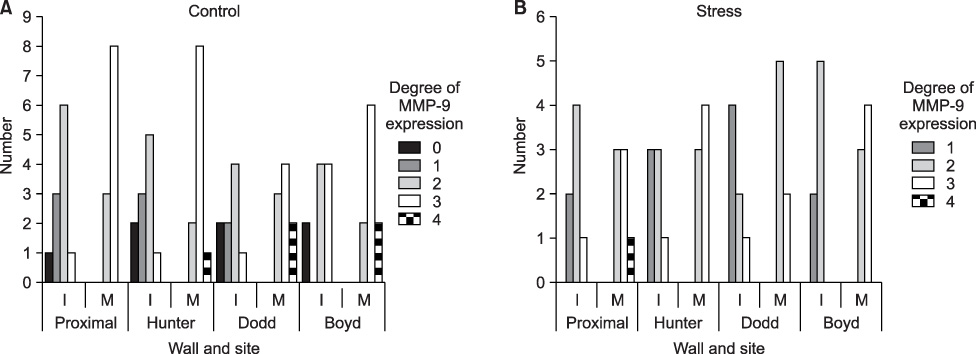
Intimal hyperplasia, fragmentation and loss of elastic fibers, infiltration of collagen fibers, and disorganization of medial muscle layers were evident in most vein samples. The degree of vein wall degeneration was not different between the 2 groups, and the anatomical sites of GSV. By immunohistochemistry, the expression of MMPs and TIMPs was not significantly different according to the group and the site. The expression of MMP-9 was more intense than that of other MMPs and TIMPs in all samples. MMP-9 was well localized to endothelial cells, medial muscle layers, and adventitial vasa vasorum.
CONCLUSION
There are no distinct differences in the varicose vein samples after short-term postural blood stasis compared to the resting group. MMP-9 may be the key enzyme of the venous wall remodeling.
MeSH Terms
Figure
Reference
-
1. Callam MJ. Epidemiology of varicose veins. Br J Surg. 1994. 81:167–173.2. Evans CJ, Fowkes FG, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health. 1999. 53:149–153.3. Pascarella L, Schmid Schonbein GW. Causes of telengiectasias, reticular veins, and varicose veins. Semin Vasc Surg. 2005. 18:2–4.4. Naoum JJ, Hunter GC, Woodside KJ, Chen C. Current advances in the pathogenesis of varicose veins. J Surg Res. 2007. 141:311–316.5. Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006. 259:381–392.6. Parra JR, Cambria RA, Hower CD, Dassow MS, Freischlag JA, Seabrook GR, et al. Tissue inhibitor of metalloproteinase-1 is increased in the saphenofemoral junction of patients with varices in the leg. J Vasc Surg. 1998. 28:669–675.7. Badier-Commander C, Verbeuren T, Lebard C, Michel JB, Jacob MP. Increased TIMP/MMP ratio in varicose veins: a possible explanation for extracellular matrix accumulation. J Pathol. 2000. 192:105–112.8. Kosugi I, Urayama H, Kasashima F, Ohtake H, Watanabe Y. Matrix metalloproteinase-9 and urokinase-type plasminogen activator in varicose veins. Ann Vasc Surg. 2003. 17:234–238.9. Woodside KJ, Hu M, Burke A, Murakami M, Pounds LL, Killewich LA, et al. Morphologic characteristics of varicose veins: possible role of metalloproteinases. J Vasc Surg. 2003. 38:162–169.10. Gillespie DL, Patel A, Fileta B, Chang A, Barnes S, Flagg A, et al. Varicose veins possess greater quantities of MMP-1 than normal veins and demonstrate regional variation in MMP-1 and MMP-13. J Surg Res. 2002. 106:233–238.11. Jacob MP, Cazaubon M, Scemama A, Prie D, Blanchet F, Guillin MC, et al. Plasma matrix metalloproteinase-9 as a marker of blood stasis in varicose veins. Circulation. 2002. 106:535–538.12. Ishikawa Y, Asuwa N, Ishii T, Ito K, Akasaka Y, Masuda T, et al. Collagen alteration in vascular remodeling by hemodynamic factors. Virchows Arch. 2000. 437:138–148.13. Patterson MA, Leville CD, Hower CD, Jean-Claude JM, Seabrook GR, Towne JB, et al. Shear force regulates matrix metalloproteinase activity in human saphenous vein organ culture. J Surg Res. 2001. 95:67–72.14. Lehoux S, Lemarie CA, Esposito B, Lijnen HR, Tedgui A. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation. 2004. 109:1041–1047.15. Pascarella L, Schmid-Schonbein GW, Bergan J. An animal model of venous hypertension: the role of inflammation in venous valve failure. J Vasc Surg. 2005. 41:303–311.16. Berceli SA, Jiang Z, Klingman NV, Schultz GS, Ozaki CK. Early differential MMP-2 and -9 dynamics during flow-induced arterial and vein graft adaptations. J Surg Res. 2006. 134:327–334.17. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006. 69:562–573.18. Fu X, Parks WC, Heinecke JW. Activation and silencing of matrix metalloproteinases. Semin Cell Dev Biol. 2008. 19:2–13.19. Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008. 75:346–359.20. Nomura S, Yoshimura K, Akiyama N, Mikamo A, Furutani A, Aoki H, et al. HMG-CoA reductase inhibitors reduce matrix metalloproteinase-9 activity in human varicose veins. Eur Surg Res. 2005. 37:370–378.21. Sansilvestri-Morel P, Rupin A, Badier-Commander C, Kern P, Fabiani JN, Verbeuren TJ, et al. Imbalance in the synthesis of collagen type I and collagen type III in smooth muscle cells derived from human varicose veins. J Vasc Res. 2001. 38:560–568.22. Sansilvestri-Morel P, Rupin A, Jaisson S, Fabiani JN, Verbeuren TJ, Vanhoutte PM. Synthesis of collagen is dysregulated in cultured fibroblasts derived from skin of subjects with varicose veins as it is in venous smooth muscle cells. Circulation. 2002. 106:479–483.23. Bergan JJ, Pascarella L, Schmid-Schonbein GW. Pathogenesis of primary chronic venous disease: Insights from animal models of venous hypertension. J Vasc Surg. 2008. 47:183–192.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Matrix Metalloproteinase-2 and -13 and Tissue Inhibitor of Metalloproteinase-4 in Varicose Veins
- Expressions of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinase in Great Saphenous Veins of Patients with Varicose Veins
- The Expression of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in the Wall of Great Saphenous Vein in Patients with Varicose Veins
- Overexpression of Estrogen Receptor in Female Patients with Varicose Vein of Lower Extremities
- Intermittent Negative Hydrostatic Pressure and Chondrocyte Metabolism