J Vet Sci.
2014 Mar;15(1):19-26. 10.4142/jvs.2014.15.1.19.
Sequential alterations of glucocorticoid receptors in the hippocampus of STZ-treated type 1 diabetic rats
- Affiliations
-
- 1Laboratory of Developmental Biology and Genomics, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- 2Department of Biomedical Laboratory Science, College of Medical Sciences, Soonchunhyang University, Asan 336-745, Korea. admiral96@sch.ac.kr
- KMID: 1737606
- DOI: http://doi.org/10.4142/jvs.2014.15.1.19
Abstract
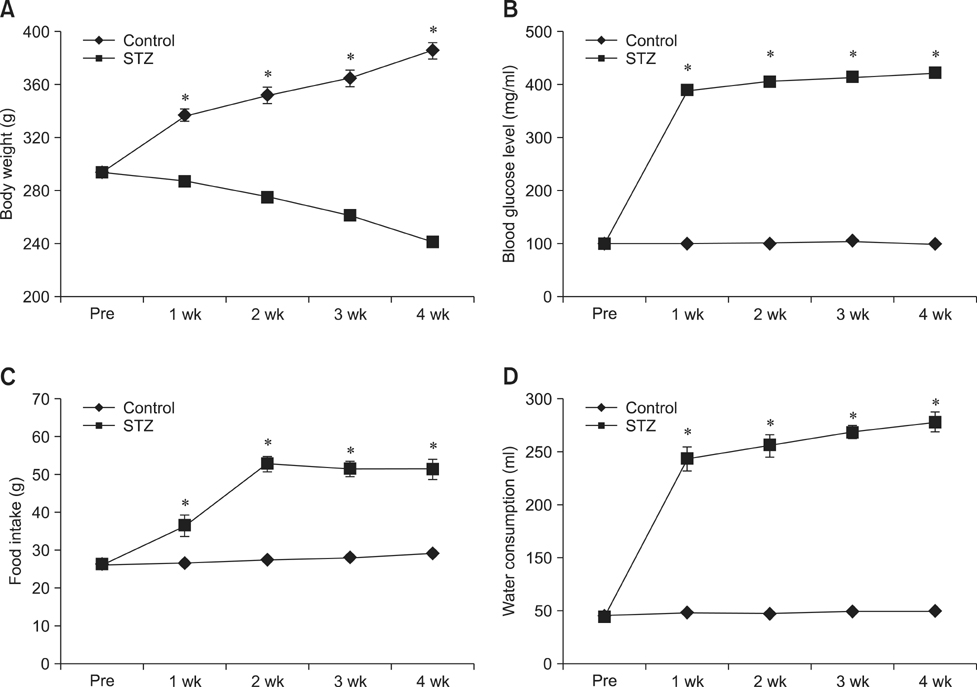
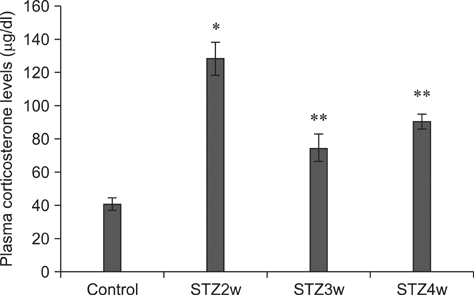
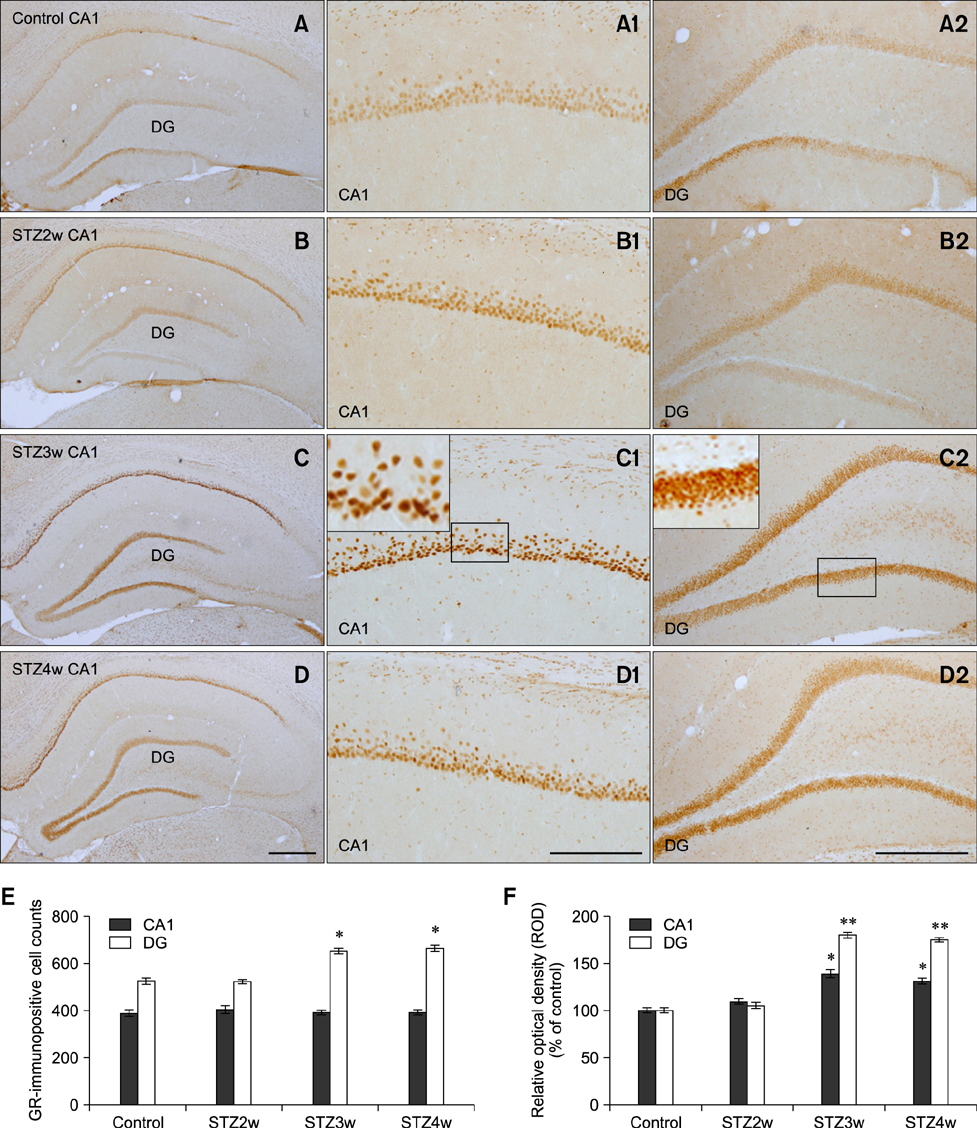
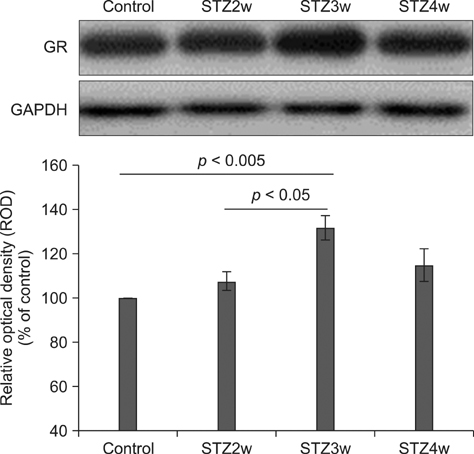
- Type 1 diabetes is a common metabolic disorder accompanied by increased blood glucose levels along with glucocorticoid and cognitive deficits. The disease is also thought to be associated with environmental changes in brain and constantly induces oxidative stress in patients. Therefore, glucocorticoid-mediated negative feedback mechanisms involving the glucocorticoid receptor (GR) binding site are very important to understand the development of this disease. Many researchers have used streptozotocin (STZ)-treated diabetic animals to study changes in GR expression in the brain. However, few scientists have evaluated the hyperglycemic period following STZ exposure. In the present study, we found GR expression in the hippocampus varied based on the period after STZ administration for up to 4 weeks. We performed immunohistochemistry and Western blotting to validate the sequential alterations of GR expression in the hippocampus of STZ-treated type 1 diabetic rats. GR protein expression increased significantly until week 3 but decreased at week 4 following STZ administration. GR expression after 70 mg/kg STZ administration was highest at 3 weeks post-treatment and decreased thereafter. Although STZ-induced increase in GR expression in diabetic animals has been described, our data indicate that researchers should consider the sequential GR expression changes during the hyperglycemic period following STZ exposure.
MeSH Terms
Figure
Reference
-
1. Ahima R, Krozowski Z, Harlan R. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J Comp Neurol. 1991; 313:522–538.
Article2. Alnemri ES, Maksymowych AB, Robertson NM, Litwack G. Overexpression and characterization of the human mineralocorticoid receptor. J Biol Chem. 1991; 266:18072–18081.
Article3. Barber M, Kasturi BS, Austin ME, Patel KP, MohanKumar SMJ, MohanKumar PS. Diabetes-induced neuroendocrine changes in rats: role of brain monoamines, insulin and leptin. Brain Res. 2003; 964:128–135.
Article4. Beauquis J, Homo-Delarche F, Giroix MH, Ehses J, Coulaud J, Roig P, Portha B, De Nicola AF, Saravia F. Hippocampal neurovascular and hypothalamic-pituitary-adrenal axis alterations in spontaneously type 2 diabetic GK rats. Exp Neurol. 2010; 222:125–134.
Article5. Bitar MS. Co-administration of etomoxir and RU-486 mitigates insulin resistance in hepatic and muscular tissues of STZ-induced diabetic rats. Horm Metab Res. 2001; 33:577–584.
Article6. Chan O, Chan S, Inouye K, Vranic M, Matthews SG. Molecular regulation of the hypothalamo-pituitary-adrenal axis in streptozotocin-induced diabetes: effects of insulin treatment. Endocrinology. 2001; 142:4872–4879.
Article7. Chan O, Inouye K, Vranic M, Matthews SG. Hyperactivation of the hypothalamo-pituitary-adrenocortical axis in streptozotocin-diabetes is associated with reduced stress responsiveness and decreased pituitary and adrenal sensitivity. Endocrinology. 2002; 143:1761–1768.
Article8. Chan O, Inouye K, Akirav E, Park E, Riddell MC, Vranic M, Matthews SG. Insulin alone increases hypothalamo-pituitary-adrenal activity, and diabetes lowers peak stress responses. Endocrinology. 2005; 146:1382–1390.
Article9. Chan O, Inouye K, Akirav EM, Park E, Riddell MC, Matthews SG, Vranic M. Hyperglycemia does not increase basal hypothalamo-pituitary-adrenal activity in diabetes but it does impair the HPA response to insulin-induced hypoglycemia. Am J Physiol Regul Integr Comp Physiol. 2005; 289:R235–R246.
Article10. De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998; 19:269–301.
Article11. Dimitriadis GD, Raptis SA. Thyroid hormone excess and glucose intolerance. Exp Clin Endocrinol Diabetes. 2001; 109:Suppl 2. S225–S239.
Article12. Erickson K, Drevets W, Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neurosci Biobehav Rev. 2003; 27:233–246.
Article13. Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-β and tau pathology in a mouse model of Alzheimer's disease. J Neurosci. 2006; 26:9047–9056.
Article14. Grillo CA, Piroli GG, Wood GE, Reznikov LR, McEwen BS, Reagan LP. Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005; 136:477–486.
Article15. Hwang IK, Yi SS, Yoo KY, Park OK, Yan B, Song W, Won MH, Yoon YS, Seong JK. Effect of treadmill exercise on blood glucose, serum corticosterone levels and glucocorticoid receptor immunoreactivity in the hippocampus in chronic diabetic rats. Neurochem Res. 2011; 36:281–287.
Article16. Jöhren O, Dendorfer A, Dominiak P, Raasch W. Gene expression of mineralocorticoid and glucocorticoid receptors in the limbic system is related to type-2 like diabetes in leptin-resistant rats. Brain Res. 2007; 1184:160–167.
Article17. McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986; 66:1121–1188.
Article18. McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995; 5:205–216.
Article19. McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999; 22:105–122.
Article20. Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998; 101:2174–2181.
Article21. Opherk C, Tronche F, Kellendonk C, Kohlmüller D, Schulze A, Schmid W, Schütz G. Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol Endocrinol. 2004; 18:1346–1353.
Article22. Paskitti ME, McCreary BJ, Herman JP. Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: time-course analysis. Brain Res Mol Brain Res. 2000; 80:142–152.
Article23. Petersson M, Uvnäs-Moberg K. Systemic oxytocin treatment modulates glucocorticoid and mineralocorticoid receptor mRNA in the rat hippocampus. Neurosci Lett. 2003; 343:97–100.
Article24. Ranhotra HS, Sharma R. Streptozotocin-induced diabetes and glucocorticoid receptor regulation: tissue- and age-specific variation. Mech Ageing Dev. 2000; 119:15–24.
Article25. Rashid S, Lewis GF. The mechanisms of differential glucocorticoid and mineralocorticoid action in the brain and peripheral tissues. Clin Biochem. 2005; 38:401–409.
Article26. Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005; 22:359–370.
Article27. Reul JMHM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985; 117:2505–2511.
Article28. Revsin Y, Rekers NV, Louwe MC, Saravia FE, De Nicola AF, de Kloet ER, Oitzl MS. Glucocorticoid receptor blockade normalizes hippocampal alterations and cognitive impairment in streptozotocin-induced type 1 diabetes mice. Neuropsychopharmacology. 2009; 34:747–758.
Article29. Thompson A, Arany EJ, Hill DJ, Yang K. Glucocorticoid receptor expression is altered in pancreatic β cells of the non-obese diabetic mouse during postnatal development. Metabolism. 2002; 51:765–768.
Article30. Viengchareun S, Penfornis P, Zennaro MC, Lombès M. Mineralocorticoid and glucocorticoid receptors inhibit UCP expression and function in brown adipocytes. Am J Physiol Endocrinol Metab. 2001; 280:E640–E649.
Article31. Yi SS, Hwang IK, Kim YN, Kim IY, Pak SI, Lee IS, Seong JK, Yoon YS. Enhanced expressions of arginine vasopressin (Avp) in the hypothalamic paraventricular and supraoptic nuclei of type 2 diabetic rats. Neurochem Res. 2008; 33:833–841.
Article32. Yi SS, Hwang IK, Chun MS, Kim YN, Kim IY, Lee IS, Seong JK, Yoon YS. Glucocorticoid receptor changes associate with age in the paraventricular nucleus of type II diabetic rat model. Neurochem Res. 2009; 34:851–858.
Article33. Yi SS, Hwang IK, Shin JH, Choi JH, Lee CH, Kim IY, Kim YN, Won MH, Park IS, Seong JK, Yoon YS. Regulatory mechanism of hypothalamo-pituitary-adrenal (HPA) axis and neuronal changes after adrenalectomy in type 2 diabetes. J Chem Neuroanat. 2010; 40:130–139.
Article34. Yi SS, Hwang IK, Kim DW, Shin JH, Nam SM, Choi JH, Lee CH, Won MH, Seong JK, Yoon YS. The chronological characteristics of SOD1 activity and inflammatory response in the hippocampi of STZ-induced type 1 diabetic rats. Neurochem Res. 2011; 36:117–128.
Article35. Yi SS. Time-dependent changes of calbindin D-28K and parvalbumin immunoreactivity in the hippocampus of rats with streptozotocin-induced type 1 diabetes. J Vet Sci. 2013; 14:373–380.
Article36. Zhe D, Fang H, Yuxiu S. Expressions of hippocampal mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) in the single-prolonged stress-rats. Acta Histochem Cytochem. 2008; 41:89–95.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Time-dependent changes of calbindin D-28K and parvalbumin immunoreactivity in the hippocampus of rats with streptozotocin-induced type 1 diabetes
- Enhanced expression of inducible nitric oxide synthase may be responsible for altered vascular reactivity in streptozotocin-induced diabetic rats
- Chlorogenic acid attenuates pro‑inflammatory response in the blood of streptozotocin‑induced diabetic rats
- Effects of Lipoxygenase Inhibitor on Diabetic Nephropathy in Rats: Decreasing Proteinuria and Preserving Renal Function
- Effects of neonatal footshock stress on glucocorticoid and 5-HT2A/2C receptor bindings and exploratory behavior