J Korean Med Sci.
2010 Jan;25(1):90-96. 10.3346/jkms.2010.25.1.90.
Immunogenicity of Haemophilus influenzae Type b Conjugate Vaccines in Korean Infants: A Meta-analysis
- Affiliations
-
- 1Department of Pediatrics, School of Medicine, Ewha Womans University, Seoul, Korea. kaykim@ewha.ac.kr
- 2Department of Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1713837
- DOI: http://doi.org/10.3346/jkms.2010.25.1.90
Abstract
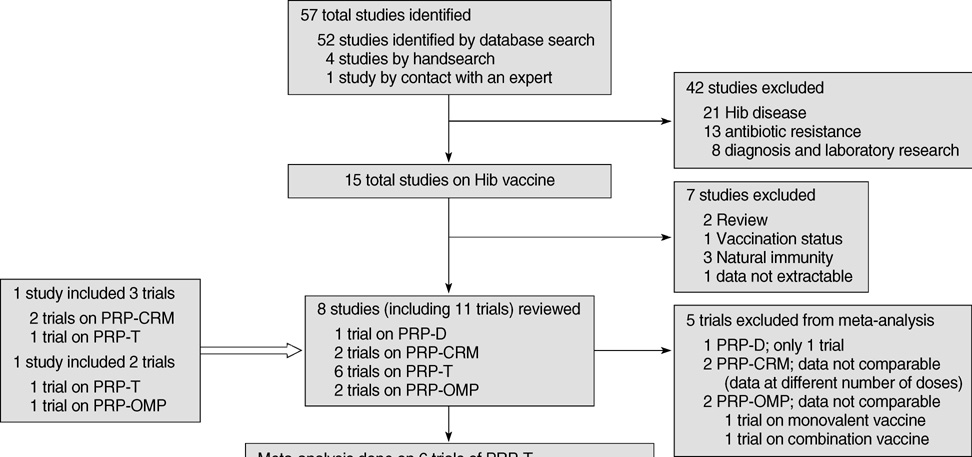
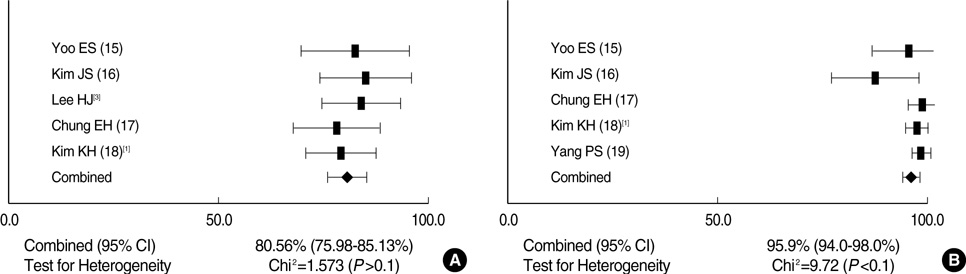
- A meta-analysis was performed on the immunogenicity of Haemophilus influenzae type b (Hib) conjugate vaccines after 2 (2 and 4 months) and 3 doses (2, 4, and 6 months) in Korean infants. A database search of MEDLINE, KoreaMed, and Korean Medical Database was done. The primary outcome measure was the proportion of infants with anti-polyribosylribitol phosphate (PRP) concentrations > or =1.0 microgram/mL. Eight studies including eleven trials were retrieved. One trial reported on the diphtheria toxoid conjugate vaccine (PRP-D) and 2 trials each on the mutant diphtheria toxin (PRP-CRM) and Neisseria meningitidis outer-membrane protein (PRP-OMP) conjugate vaccine. Heterogeneity in study designs between trials on PRP-CRM was noted and one trial reported on a monovalent and another on a combination PRP-OMP vaccine. Thus, a meta-analysis was conducted only on the tetanus toxoid conjugate vaccine (PRP-T). After a primary series of 2 doses and 3 doses, 80.6% (95% confidence interval [CI]; 76.0-85.1%) and 95.7% (95% CI; 94.0-98.0%) of infants achieved an antibody level > or =1.0 microgram/mL, respectively. The immunogenic response to the PRP-T vaccine was acceptable after a primary series of 3 doses and also 2 doses. A reduced number of doses as a primary series could be carefully considered in Korean infants.
MeSH Terms
Figure
Reference
-
1. Wenger JD, Ward WJ. Plotkin SA, Orenstein WA, editors. Haemophilus influenzae vaccine. Vaccines. 2004. 4th ed. Philadelphia: WB Saunders Co;229–268.2. Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000. 13:302–317.3. Heath PT. Haemophilus influenzae type b conjugate vaccines: a review of efficacy data. Pediatr Infect Dis J. 1998. 17:9 Suppl. S117–S122.4. Decker MD, Edwards KM, Bradley R, Palmer P. Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccines. J Pediatr. 1992. 120:184–189.5. Obonyo CO, Lau J. Efficacy of Haemophilus influenzae type b vaccination of children: a meta-analysis. Eur J Clin Microbiol Infect Dis. 2006. 25:90–97.6. Eskola J, Kayhty H, Takala AK, Peltola H, Ronnberg PR, Kela E, Pekkanen E, McVerry PH, Makela PH. A randomized, prospective field trial of a conjugate vaccine in the protection of infants and young children against invasive Haemophilus influenzae type b disease. N Engl J Med. 1990. 323:1381–1387.7. Kayhty H, Peltola H, Karanko V, Makela PH. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983. 147:1100.8. Centers for Disease Control and Prevention (CDC). Progress toward elimination of Haemophilus influenzae type b invasive disease among infants and children--United States, 1998-2000. MMWR Morb Mortal Wkly Rep. 2002. 51:234–237.9. World Health Organization. WHO position paper on Haemophilus influenzae type b conjugate vaccines. (Replaces WHO position paper on Hib vaccines previously published in the Weekly Epidemiological Record. Wkly Epidemiol Rec. 2006. 81:445–452.10. Rossi IA, Zuber PL, Dumolard L, Walker DG, Watt J. Introduction of Hib vaccine into national immunization programmes: a descriptive analysis of global trends. Vaccine. 2007. 25:7075–7080.11. Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care. 1990. 6:5–30.12. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986. 7:177–188.13. Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Metaanalyses. Lancet. 1999. 354:1896–1900.14. Choi SY, Kim HT, Kim YW, Kang YJ, Chung YC, Chang JK, Lee HJ. Immunogenicity of Haemophilus influenzae PRP-D conjugate vaccine in Korean infants. J Korean Pediatr Soc. 1999. 42:771–777.15. Yoo ES, Park EA, Kim GH. Natural anti-PRP antibody levels of Haemophilus influenzae type b (Hib) and changes of antibody levels after three doses of vaccination. J Korean Pediatr Soc. 1995. 38:1201–1209.16. Kim JS, Cho SB, Lee HR, Park SK, Hwang PH. Immunogenicity and safety of Haemophilus influenzae type b polysaccharide-tetanus toxoid conjugate vaccine (PRP-T) in Korean infants. Korean J Infect Dis. 1996. 28:225–232.17. Chung EH, Kim YJ, Kim YK, Kim DH, Seo JW, Lee HJ. Immunogenicity and safety of a Haemophilus influenzae type b polysaccharide-tetanus toxoid conjugate vaccine (PTP-T; Hiberix™) in Korean infants. Korean J Pediatr Infect Dis. 2003. 10:71–79.18. Kim KH, Lee H, Chung EH, Kang JH, Kim JH, Kim JS, Lee HJ, Oh SH, Park EA, Park SE. Immunogenicity and safety of two Haemophilus influenza type b conjugate vaccines in Korean infants. J Korean Med Sci. 2008. 23:929–936.19. Yang PS, Seo JI, Noh KT, Yoo JH, Hwang KS, Hwang KG. Studies of the change of antibody titers after vaccination of Haemophilus influenzae PRP-T conjugate vaccine. J Korean Pediatr Soc. 2002. 45:987–993.20. Chung EH, Ma SH, Hong YJ, Kim KH, Kim JH, Lee JA, Lee HJ. Immunogenicity and safety of a combined Hepatitis B and Haemophilus influenza Type b conjugate (PRP-OMP) vaccine (Comvax™; Merck & Co.) in Korean infants. Korean J Pediatr Infect Dis. 2006. 13:163–173.21. Lagos R, Valenzuela MT, Levine OS, Losonsky GA, Erazo A, Wasserman SS, Levine MM. Economisation of vaccination against Haemophilus influenzae type b: a randomised trial of immunogenicity of fractional-dose and two-dose regimens. Lancet. 1998. 351:1472–1476.22. Mulholland K, Hilton S, Adegbola R, Usen S, Oparaugo A, Omosigho C, Weber M, Palmer A, Schneider G, Jobe K, Lahai G, Jaffar S, Secka O, Lin K, Ethevenaux C, Greenwood B. Randomised trial of Haemophilus influenzae type-b tetanus protein conjugate vaccine [corrected] for prevention of pneumonia and meningitis in Gambian infants. Lancet. 1997. 349:1191–1197.23. Santosham M, Wolff M, Reid R, Hohenboken M, Bateman M, Goepp J, Cortese M, Sack D, Hill J, Newcomer W, Capriotti L, Smith J, Owen M, Gahagan S, Hu D, Kling R, Lukacs L, Ellis RW, Vella PP, Calandra G, Matthews H, Ahonkhai V. The efficacy in Navajo infants of a conjugate vaccine consisting of Haemophilus influenzae type b polysaccharide and Neisseria meningitidis outer-membrane protein complex. N Engl J Med. 1991. 324:1767–1772.24. Peltola H, Aavitsland P, Hansen KG, Jonsdottir KE, Nokleby H, Romanus V. Perspective: a five-country analysis of the impact of four different Haemophilus influenzae type b conjugates and vaccination strategies in Scandinavia. J Infect Dis. 1999. 179:223–229.25. Kurikka S, Kayhty H, Saarinen L, Ronnberg P, Eskola J, Makela PH. Comparison of five different vaccination schedules with Haemophilus influenzae type b-tetanus toxoid conjugate vaccine. J Pediatr. 1996. 128:524–530.26. Kim JS, Jang YT, Kim JD, Park TH, Park JM, Kilgore PE, Kennedy WA, Park E, Nyambat B, Kim DR, Hwang PH, Kim SJ, Eun SH, Lee HS, Cho JH, Kim YS, Chang SJ, Huang HF, Clemens JD, Ward JI. Incidence of Haemophilus influenzae type b and other invasive diseases in South Korean children. Vaccine. 2004. 22:3952–3962.27. Kelly DF, Moxon ER, Pollard AJ. Haemophilus influenzae type b conjugate vaccines. Immunology. 2004. 113:163–174.28. Makela PH, Kayhty H, Leino T, Auranen K, Peltola H, Ekstrom N, Eskola J. Long-term persistence of immunity after immunisation with Haemophilus influenzae type b conjugate vaccine. Vaccine. 2003. 22:287–292.29. Anderson P. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1984. 149:1034–1035.30. Granoff DM. Assessing efficacy of Haemophilus influenzae type b combination vaccines. Clin Infect Dis. 2001. 33:Suppl 4. S278–S287.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Present status and prospects of Haemophilus influenzae type b(Hib) immunization
- Immunogenicity and Safety of Two Different Haemophilus influenzae Type b Conjugate Vaccines in Korean Infants
- Immunogenicity of Haemophilus influenzae PRP-D Conjugate Vaccine in Korean Infants
- Immunogenicity and Safety of a Haemophilus influenzae Type b Polysaccharide-Tetanus Toxoid Conjugate Vaccine (PRP-T; Hiberixâ„¢) in Korean Infants
- A Case of Neurologic Sequelae and a Case of Peripheral Gangrene of Extremities Associated with Haemophilus influenzae Type b Meningitis