Improved Antibiotic Susceptibility Test of Orientia tsutsugamushi by Flow Cytometry Using Monoclonal Antibody
- Affiliations
-
- 1Department of Microbiology, Center for Advanced Medical Education by BK21 Project, and Research Institute for Medical Science, Inha University College of Medicine, Incheon, Korea. jaeskang@inha.ac.kr
- KMID: 1713220
- DOI: http://doi.org/10.3346/jkms.2007.22.1.1
Abstract
- Orientia tsutsugamushi causes scrub typhus, which is endemic in many countries in the Asia-Pacific region including Korea. Recent emergence of doxycycline-resistant strains from Thailand has underlined the importance of the susceptibility tests of O. tsutsugamushi to antibiotics. To improve the flow cytometric technique for the susceptibility test, we applied a monoclonal antibody (MAb) in the quantification of O. tsutsugamushi. With using MAb FS15, we determined the doxycycline susceptibility of two strains, Boryong and AFSC-4 strain which is reported to be doxycycline-sensitive and resistant, respectively. The growth of both strains was inhibited to below 10% of the control in the presence of 0.1 microgram/mL or higher concentrations of doxycycline. We suggest that our approach is more quantitative and reproducible than the conventional microscopic methods.
Keyword
MeSH Terms
Figure
Cited by 10 articles
-
In Vitro Activity of Tigecycline Against Orientia tsutsugamushi
Sun-Myoung Lee, Hae-Yoon Kwon, Jae-Hyoung Im, Ji Hyeon Baek, Seung-Sik Hwang, Jae-Seung Kang, Moon-Hyun Chung, Jin-Soo Lee
Yonsei Med J. 2016;57(4):1034-1037. doi: 10.3349/ymj.2016.57.4.1034.In Vitro Antibiotic Susceptibility of Orientia tsutsugamushi strain Boryong Measured by Flow Cytometry
Eun Sil Kim, Mee Kyung Kim, Hye Myung Lee, Moon-Hyun Chung, Jin-Soo Lee, Jae Eun Park, Jae-Seung Kang
Infect Chemother. 2008;40(4):212-217. doi: 10.3947/ic.2008.40.4.212.In Vitro Antibiotic Susceptibility of Orientia tsutsugamushi strain Boryong Measured by Flow Cytometry
Eun Sil Kim, Mee Kyung Kim, Hye Myung Lee, Moon-Hyun Chung, Jin-Soo Lee, Jae Eun Park, Jae-Seung Kang
Infect Chemother. 2008;40(4):212-217. doi: 10.3947/ic.2008.40.4.212.A Case of Scrub Typhus in Summer Presenting as Atypical Pneumonia
Sang Don Park, Moon-Hyun Chung, Hye Myung Lee, Mee-Kyung Kim, Jae-Seung Kang
Infect Chemother. 2008;40(4):241-245. doi: 10.3947/ic.2008.40.4.241.In vitro Study on the Dose and Duration of Doxycycline Treatment against Orientia tsutsugamushi
Eun Sil Kim, Mee Kyung Kim, Hye Myung Lee, Moon-Hyun Chung, Jin-Soo Lee, Jae Eun Park, Jae-Seung Kang
Infect Chemother. 2008;40(5):249-254. doi: 10.3947/ic.2008.40.5.249.Doxycycline Resistance in Orientia tsutsugamushi Isolated from Korean Patients
Eun Sil Kim, Mee Kyung Kim, Hye Myung Lee, Se-Hee Kil, Moon-Hyun Chung, Jin-Soo Lee, Jae-Seung Kang
Infect Chemother. 2008;40(5):259-265. doi: 10.3947/ic.2008.40.5.259.In vitro Efficacy of Antibiotic Combinations against Orientia tsutsugamushi
Eun Sil Kim, Mee Kyung Kim, Hye Myung Lee, Moon-Hyun Chung, Jin-Soo Lee, Jae-Seung Kang
Infect Chemother. 2008;40(6):311-315. doi: 10.3947/ic.2008.40.6.311.Cholestatic Hepatitis Caused by Tongyeong Strain of Orientia tsutsugamushi
Kowoon Joo, Mee Kyung Kim, Se-Hee Kil, Moon-Hyun Chung, Joon-Mee Kim, Jae-Seung Kang
Infect Chemother. 2009;41(2):99-104. doi: 10.3947/ic.2009.41.2.99.High In Vitro Infectivity of a Doxycycline-Insensitive Strain of Orientia tsutsugamushi
Min Su Kim, Ji Hyeon Baek, Jin-Soo Lee, Moon-Hyun Chung, Sun Myoung Lee, Jae-Seung Kang
Infect Chemother. 2013;45(4):431-434. doi: 10.3947/ic.2013.45.4.431.In vitro Synergism between Chloroquine and Antibiotics against Orientia tsutsugamushi
Dongwook Son, Moon-Hyun Chung
Infect Chemother. 2014;46(3):182-188. doi: 10.3947/ic.2014.46.3.182.
Reference
-
1. Seong SY, Choi MS, Kim IS. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect. 2001. 3:11–21.2. Chang WH. Current status of tsutsugamushi disease in Korea. J Korean Med Sci. 1995. 10:227–238.
Article3. Moron CG, Popov VL, Feng HM, Wear D, Walker DH. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod Pathol. 2001. 14:752–759.
Article4. Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, Jongsakul K, Teja-Isavadharm P, Bhodhidatta D, Corcoran KD, Dasch GA, Strickman D. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet. 1996. 348:86–89.
Article5. Strickman D, Sheer T, Salata K, Hershey J, Dasch G, Kelly D, Kuschner R. In vitro effectiveness of azithromycin against doxycycline-resistant and -susceptible strains of Rickettsia tsutsugamushi, etiologic agent of scrub typhus. Antimicrob Agents Chemother. 1995. 39:2406–2410.
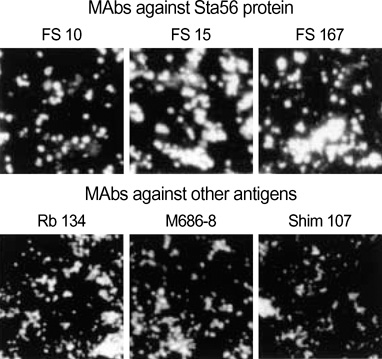
Article6. Kim MK, Odgerel Z, Chung MH, Lim BU, Kang JS. Characterization of monoclonal antibody reacting exclusively against intracellular Orientia tsutsugamushi. Microbiol Immunol. 2002. 46:733–740.7. Kim MK, Odgerel Z, Kim MJ, Chung MH, Lim BU, Kang JS. Application of monoclonal antibody, specific for intracellular Orientia tsutsugamushi, to immunofluorescent antibody test for determining antibiotic susceptibility. Microbiol Immunol. 2004. 48:655–660.8. Alvarez-Barrientos A, Arroyo J, Canton R, Nombela C, Sanchez-Perez M. Applications of flow cytometry to clinical microbiology. Clin Microbiol Rev. 2000. 13:167–195.
Article9. Dessus-Babus S, Belloc F, Bebear CM, Poutiers F, Lacombe F, Bebear C, de Barbeyrac B. Antibiotic susceptibility testing for Chlamydia trachomatis using flow cytometry. Cytometry. 1998. 31:37–44.10. Levitt D, Zable B, Bard J. Binding, ingestion, and growth of Chlamydia trachomatis (L2 serovar) analyzed by flow cytometry. Cytometry. 1986. 7:378–383.11. Matyszak MK, Young JL, Gaston JS. Uptake and processing of Chlamydia trachomatis by human dendritic cells. Eur J Immunol. 2002. 32:742–751.
Article12. Waldman FM, Hadley WK, Fulwyler MJ, Schachter J. Flow cytometric analysis of Chlamydia trachomatis interaction with L cells. Cytometry. 1987. 8:55–59.13. Messick JB, Rikihisa Y. Characterization of Ehrlichia risticii binding, internalization, and proliferation in host cells by flow cytometry. Infect Immun. 1993. 61:3803–3810.
Article14. Kelly DJ, Salata KF, Strickman D, Hershey JN. Rickettsia tsutsugamushi infection in cell culture: antibiotic susceptibility determined by flow cytometry. Am J Trop Med Hyg. 1995. 53:602–606.
Article15. Chang WH, Kang JS, Lee WK, Choi MS, Lee JH. Serological classification by monoclonal antibodies of Rickettsia tsutsugamushi isolated in Korea. J Clin Microbiol. 1990. 28:685–688.
Article16. Kim MK, Kee SH, Cho KA, Chung MH, Lim BU, Chang WH, Kang JS. Apoptosis of endothelial cell line ECV304 persistently infected with Orientia tsutsugamushi. Microbiol Immunol. 1999. 43:751–757.17. Seong SY, Kim MK, Lee SM, Odgerel Z, Choi MS, Han TH, Kim IS, Kang JS, Lim BU. Neutralization epitopes on the antigenic domain II of the Orientia tsutsugamushi 56-kDa protein revealed by monoclonal antibodies. Vaccine. 2000. 19:2–9.
Article18. Kang JS, Chang WH. Antigenic relationship among the eight prototype and new serotype strains of Orientia tsutsugamushi revealed by monoclonal antibodies. Microbiol Immunol. 1999. 43:229–234.19. Spector DL, Fu XD, Maniatis T. Associations between distinct premRNA splicing components and the cell nucleus. EMBO J. 1991. 10:3467–3481.
Article20. Raoult D, Roussellier P, Vestris G, Tamalet J. In vitro antibiotic susceptibility of Rickettsia rickettsii and Rickettsia conorii: plaque assay and microplaque colorimetric assay. J Infect Dis. 1987. 155:1059–1062.
Article21. Hanson B. Improved plaque assay for Rickettsia tsutsugamushi. Am J Trop Med Hyg. 1987. 36:631–638.
Article22. Weiss E. Growth and physiology of Rickettsiae. Bacteriol Rev. 1973. 37:259–283.
Article23. Kim SW, Ihn KS, Han SH, Seong SY, Kim IS, Choi MS. Microtubule and dynein-mediated movement of Orientia tsutsugamushi to the microtubule organizing center. Infect Immun. 2001. 69:494–500.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vitro Efficacy of Antibiotic Combinations against Orientia tsutsugamushi
- Analysis of antigenic characteristics of Rickettsia tsutsugamushi Boryong strain and antigenic heterogeneity of Rickettsia tsutsugamushi using monoclonal antibodies
- Differentially Expressed Antigens of Orientia tsutsugamushi Revealed by Monoclonal Antibodies
- In Vitro Antibiotic Susceptibility of Orientia tsutsugamushi strain Boryong Measured by Flow Cytometry
- Doxycycline Resistance in Orientia tsutsugamushi Isolated from Korean Patients