J Korean Med Sci.
2006 Dec;21(6):1060-1063. 10.3346/jkms.2006.21.6.1060.
Effects of Bladder Training and/or Tolterodine in Female Patients with Overactive Bladder Syndrome: A Prospective, Randomized Study
- Affiliations
-
- 1Department of Urology, University of Ulsan College of Medicine, Asan Medical Center, 388-1 Poongnapdong, Songpa-gu, Seoul, Korea. mschoo@amc.seoul.kr
- 2Department of Urology, Seoul Adventist Hospital, Seoul, Korea.
- 3Department of Urology, Sungkyunkwan University School of Medicine, Samsung Medical Center, Seoul, Korea.
- KMID: 1713119
- DOI: http://doi.org/10.3346/jkms.2006.21.6.1060
Abstract
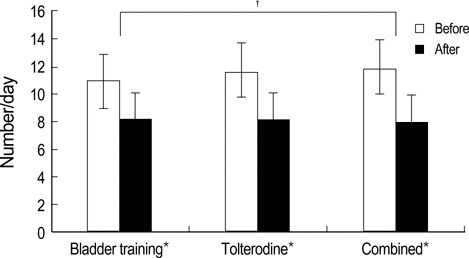
- We compared the effects of bladder training and/or tolterodine as first line treatment in female patients with overactive bladder (OAB). One hundred and thirty-nine female patients with OAB were randomized to treatment with bladder training (BT), tolterodine (To, 2 mg twice daily) or both (Co) for 12 weeks. Treatment efficacy was measured by micturition diary, urgency scores and patients' subjective assessment of their bladder condition. Mean frequency and nocturia significantly decreased in all treatment groups, declining 25.9% and 56.1%, respectively, in the BT group; 30.2% and 65.4%, respectively, in the To group; and 33.5% and 66.3%, respectively in the Co group (p<0.05 for each). The decrease in frequency was significantly greater in the Co group than in the BT group (p<0.05). Mean urgency score decreased by 44.8%, 62.2% and 60.2% in the BT, To, and Co groups, respectively, and the improvement was significantly greater in the To and Co groups than in the BT group (p<0.05 for each). Although BT, To and their combination were all effective in controlling OAB symptoms, combination therapy was more effective than either method alone. Tolterodine alone may be instituted as a first-line therapy, but may be more effective when combined with bladder training.
MeSH Terms
-
Urinary Bladder, Overactive/*therapy
Treatment Outcome
Toilet Training
Phenylpropanolamine/*therapeutic use
Outcome Assessment (Health Care)
Muscarinic Antagonists/therapeutic use
Middle Aged
Humans
Female
Cresols/*therapeutic use
Combined Modality Therapy
Benzhydryl Compounds/*therapeutic use
Behavior Therapy/*methods
Figure
Reference
-
1. Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001. 87:760–766.
Article2. Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, Wein AJ. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003. 20:327–336.
Article3. Blok BF, Holstege G. The central control of micturition and continence: implications for urology. BJU Int. 1999. 83:1–6.
Article4. Igawa Y. Discussion: functional role of M1, M2, and M3 muscarinic receptors in overactive bladder. Urology. 2000. 55:47–50.
Article5. Fitzpatrick JM. Facts and future lines of research in lower urinary tract symptoms in men and women: an overview of the role of α1-adrenoreceptor antagonists. BJU Int. 2000. 85:1–5.6. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A. Standardisation Subcommittee of the International Continence Society. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002. 21:167–178.
Article7. Artibani W. Diagnosis and significance of idiopathic overactive bladder. Urology. 1997. 50:25–32.
Article8. Frewen WK. The management of urgency and frequency of micturition. Br J Urol. 1982. 52:367–369.
Article9. Elder DD, Stephenson TP. An assessment of the Frewen regime in the treatment of detrusor dysfunction in females. Br J Urol. 1980. 52:467–471.
Article10. Bo K, Berghmans LC. Nonpharmacologic treatments for overactive bladder-pelvic floor exercises. Urology. 2000. 55:7–11.
Article11. Burgio KL, Locher JL, Goode PS, Hardin JM, McDowell BJ, Dombrowski M, Candib D. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA. 1998. 280:1995–2000.
Article12. Godec C, Cass AS, Ayala GF. Bladder inhibition with functional electrical stimulation. Urology. 1975. 6:663–666.
Article13. Jeffcoate TN, Francis WJ. Urgency incontinence in the female. Am J Obstet Gynecol. 1966. 94:604–618.
Article14. Pengelly AW, Booth CM. A prospective trial of bladder training as treatment for detrusor instability. Br J Urol. 1980. 52:463–466.
Article15. Holmes DM, Stone AR, Bary PR, Richards CJ, Stephenson TP. Bladder training-3 years on. Br J Urol. 1983. 55:660–664.
Article16. Jarvis GJ. A controlled trial of bladder drill and drug therapy in the management of detrusor instability. Br J Urol. 1981. 53:565–566.
Article17. Millard RJ. Clinical efficacy of tolterodine with or without a simplified pelvic floor exercise regimen. Neurourol Urodyn. 2004. 23:48–53.
Article18. Mattiasson A, Blaakaer J, Hoye K, Wein AJ. Tolterodine Scandinavian Study Group. Simplified bladder training augments the effectiveness of tolterodine in patients with an overactive bladder. BJU Int. 2003. 91:54–60.
Article19. Messelink EJ. Treatment of the overactive bladder with tolterodine, a new muscarinic receptor antagonist. BJU Int. 1999. 83:48–52.
Article20. Appell RA. Clinical efficacy and safety of tolterodine in the treatment of overactive bladder: a pooled analysis. Urology. 1997. 50:90–96.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Bladder Training, Tolterodine and Bladder Training with Tolterodine in Female Patients with Overactive Bladder: Prospective, Randomized Study
- The Change of Cognitive Function after Administration of Tolterodine in Brain Disease Patients with Overactive Bladder
- Review of the Anticholinergics for the Treatment of Overactive Bladder: 2009 Update
- The Effect of tolterodine Via Oral and Intravenous Administrations on Voiding in Awake Spontaneously Hypertensive Rats as an Overactive Bladder Model
- Overactive Bladder