J Korean Med Sci.
2005 Apr;20(2):297-301. 10.3346/jkms.2005.20.2.297.
Biodegradable Polymer Releasing Antibiotic Developed for Drainage Catheter of Cerebrospinal Fluid:In Vitro Results
- Affiliations
-
- 1Department of Neurosurgery, School of Medicine Ajou University, Suwon, Korea. ee80@ajou.ac.kr
- 2Center for Advanced Functional Polymers, Department of Polymer Science, Sungkyunkwan University, Suwon, Korea.
- 3Department of Engineering, Sungkyunkwan University, Suwon, Korea.
- 4Department of Neurosurgery, Asan Hospital, Seoul, Korea.
- KMID: 1095207
- DOI: http://doi.org/10.3346/jkms.2005.20.2.297
Abstract
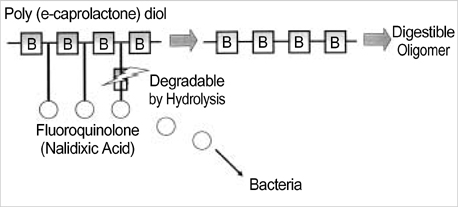
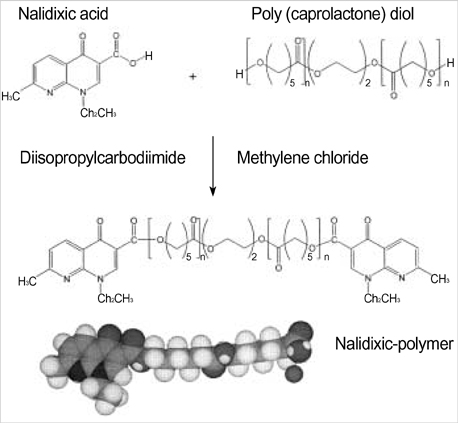
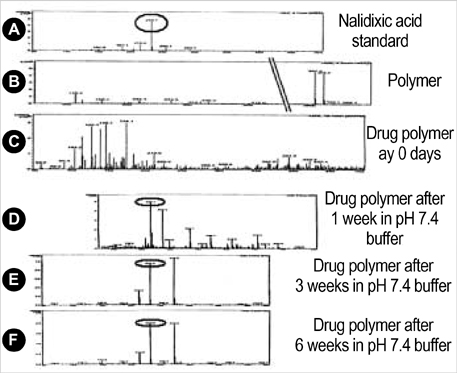
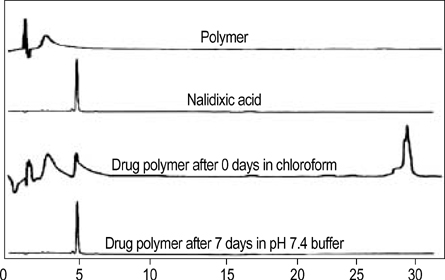
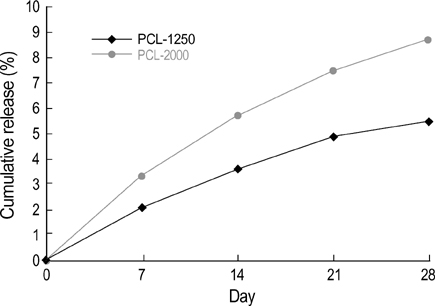
- The authors developed a biodegradable polymer that releases an antibiotic (nalidixic acid) slowly and continuously, for prevention of catheter-induced infection during drainage of cerebrospinal fluid. We investigated the in vitro antibiotic releasing characteristics and bacterial killing effects of the new polymer against E. coli. The novel fluoroquinolone polymer was prepared using diisopropylcarbodiimide, poly (e-capro-lactone) diol, and nalidixic acid. FT-IR, mass spectrometry, and elemental analysis proved that the novel antibacterial polymer was prepared successfully without any side products. Negative MS showed that the released drug has a similar molecular weight (M.W.=232, 350) to pure drug (M.W.=232). In high pressure liquid chromatography, the released drug and drug-oligomer showed similar retention times (about 4.5-5 min) in comparison to pure drug (4.5 min). The released nalidixic acid and nalidixic acid derivatives have antibacterial characteristics against E. Coli, Staphylococcus aureus, and Salmonella typhi, of more than 3 months duration. This study suggests the possibility of applying this new polymer to manufacture drainage catheters that resist catheter-induced infection, by delivering antibiotics for a longer period of more than 1 month.
Keyword
MeSH Terms
-
Anti-Bacterial Agents/*administration & dosage
Biodegradation
Biofilms
Catheterization/*adverse effects
Cerebrospinal Fluid/*physiology
Chromatography, High Pressure Liquid
Drainage/*adverse effects
*Drug Delivery Systems
Humans
Nalidixic Acid/*administration & dosage
Polymers/administration & dosage
Research Support, Non-U.S. Gov't
Spectrum Analysis, Mass
Figure
Reference
-
1. Holloway KL, Barnes T, Choi S, Bullock R, Marshall LF, Eisenberg HM, Jane JA, Ward JD, Young HF, Marmarou A. Ventriculostomy infections: the effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg. 1996. 85:419–424.
Article2. Mayhall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD, Narayan RK. Ventriculostomy-related infections. A prospective epidemiologic study. N Engl J Med. 1984. 310:553–559.
Article3. Stenager E, Gerner-Smidt P, Kock-Jensen C. Ventriculostomy-related infections-an epidemiological study. Acta Neurochir (Wien). 1986. 83:20–23.
Article4. Friedman WA, Vries JK. Percutaneous tunnel ventriculostomy: Summary of 100 procedures. J Neurosurg. 1980. 53:662–665.5. Hamilton AJ, Orozco J, Narotam P, Bowersock T. Efficacy of vancomycin/tri-iododecyclemethyl ammonium chloride-coated ventriculostomy catheters in reducing infection. Neurosurgery. 1997. 40:1043–1049.
Article6. Khanna RK, Rosenblum ML, Rock JP, Malik GM. Prolonged external ventricular drainage with percutaneous long-tunnel ventriculostomies. J Neurosurg. 1995. 83:791–794.
Article7. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000. 132:391–402.
Article8. Mermel LA, Stolz SM, Maki DG. Surface antimicrobial activity of heparin bonded and antiseptic impregnated vascular catheters. J Infect Dis. 1993. 167:920–924.9. Kwok CS, Wan C, Hendricks S, Bryers JD, Horbett TA, Ratner BD. Design of infection-resistant antibiotic-releasing polymers: I. Fabrication and formulation. J Control Release. 1999. 62:289–299.
Article10. Woo GL, Mittelman MW, Santerre JP. Synthesis and characterization of a novel biodegradable antimicrobial polymer. Biomaterials. 2000. 21:1235–1246.
Article11. Bayston R, Milner RD. Antimicrobial activity of silicone rubber used in hydrocephalus shunts after impregnation with antimicrobial substances. J Clin Pathol. 1981. 34:1057–1062.
Article12. Schierholz J, Jansen B, Jaenicke L, Pulverer G. In-vitro efficacy of an antibiotic releasing silicone ventricle catheter to prevent shunt infection. Biomaterials. 1994. 15:996–1000.
Article13. Bach A, Bohrer H, Motsch J, Martin E, Geiss HK, Sonntag HG. Prevention of bacterial colonization of intravenous catheters by antiseptic impregnation of polyurethane polymers. J Antimicrob Chemother. 1994. 33:969–978.
Article14. Darouiche RO, Raad II, Heard SO, Thornby JI, Wenker OC, Garbrielli A, Berg J, Khardori N, Hanna H, Hachem R, Harris RL, Mayhall G. A comparison of two antimicrobial-impregnated central venous catheters. N Engl J Med. 1999. 340:1–8.
Article15. Jansen B, Jansen S, Peters G, Pulverer G. In vitro effcacy of a central venous catheter ('Hydrocath') loaded with teicoplanin to prevent bacterial colonization. J Hosp Infect. 1992. 22:93–107.16. Zabramski JM, Whiting D, Darouiche RO, Horner TG, Olson J, Robertson C, Hamilton AJ. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. 2003. 98:725–730.
Article17. Buret A, Ward KH, Olson ME, Costerton JW. An in vivo model to study the pathobiology of infectious biofilms on biomaterial surfaces. J Biomed Mater Res. 1991. 25:865–874.18. Costerton JW. Bacterial biofilms in nature and disease. Ann Rev Microbiol. 1987. 41:435–464.
Article19. Costerton JW, Lewandowski Z, Caldwell D, Korber DR, Lapin-Scott HM. Microbial biofilms. Ann Rev Microbiol. 1995. 49:711–745.
Article20. Anwar H, Strap JL, Costerton JW. Establishment of aging biofilms: possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrob Agents Chemother. 1992. 36:1347–1351.
Article21. Gray ED, Peters G, Verstegen M, Regelmann WE. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet. 1984. 1:365–367.
Article22. Raad I, Darouiche R, Hachem R, Sacilowski M, Bodey GP. Antibiotics and prevention of microbial colonization of catheters. Antimicrob Agents Chemother. 1995. 39:2397–2400.
Article23. Arizono T, Oga M, Sugioka Y. Increased resistance of bacteria after adherence to polymethyl methacrylate. An in vitro study. Acta Orthop Scand. 1992. 63:661–664.24. Sherertz RJ, Forman MD, Solomon DD. Efficacy of dicloxacillin coated polyurethane catheters in preventing subcutaneous Staphylococcus aureus infection in mice. Antimicrob Agents Chemother. 1989. 33:1174–1178.25. Dasgupta MK. Silver-coated catheters in peritoneal dialysis. Perit Dial Int. 1997. 17:S142–S145.
Article26. Jansen B, Kohnen W. Prevention of biofilm formation by polymer modification. J Ind Microbiol. 1995. 15:391–396.
Article27. Levy ML, Luu T, Meltzer HS, Bennett R, Bruce DA. Bacterial adhesion to surfactant-modified silicone surfaces. Neurosurgery. 2004. 54:488–490.
Article28. Sioshansi P. New processes for surface treatment of catheters. Artif Organs. 1994. 18:266–271.
Article29. Pemberton LB, Ross V, Cuddy P, Kremer H, Fessler T, McGurk E. No difference in catheter sepsis between standard and antiseptic central venous catheters. A prospective randomized trial. Arch Surg. 1996. 131:986–989.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antibiotic Concentrations of Cerebrospinal fluid in Patients with Various Amount of Extraventricular Drainage during Intraventricular Infusion
- Shearing of an intrathecal catheter during insertion for cerebrospinal fluid drainage
- Usefulness of an Additional Mattress Suture for the Extracranial Drainage Catheter
- Remote Hemorrhage after Burr Hole Drainage of Chronic Subdural Hematoma
- Burr Hole Drainage : Could Be Another Treatment Option for Cerebrospinal Fluid Leakage after Unidentified Dural Tear during Spinal Surgery?