J Vet Sci.
2007 Jun;8(2):169-174. 10.4142/jvs.2007.8.2.169.
Suppressive effect of culture supernatant of erythrocytes and serum from dogs infected with Babesia gibsoni on the morphological maturation of canine reticulocytes in vitro
- Affiliations
-
- 1Laboratory of Veterinary Surgery, College of Veterinary Medicine, Chungbuk National University, Chungju 361-763, Korea. mdalamgir_hossain@yahoo.com
- 2Laboratory of Clinical Pathology, Department of Veterinary Clinical Sciences, Faculty of Agriculture, Kagoshima University, 1-21-24 Kohrimoto, Kagoshima 890-0065, Japan.
- 3Laboratory of Internal Medicine, Department of Veterinary Clinical Sciences, Graduate School of Veterinary Medicine, Hokkaido University, Sapporo 060-0818, Japan.
- 4Department of Pathology and Parasitology, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong 4202, Bangladesh. mdalamgir_hossain@yahoo.com
- KMID: 1089669
- DOI: http://doi.org/10.4142/jvs.2007.8.2.169
Abstract
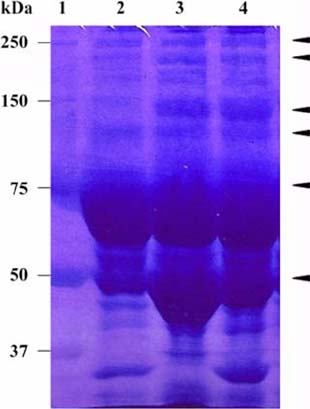
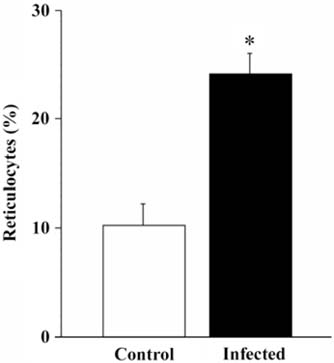
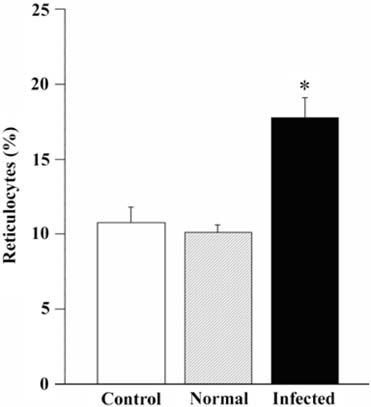
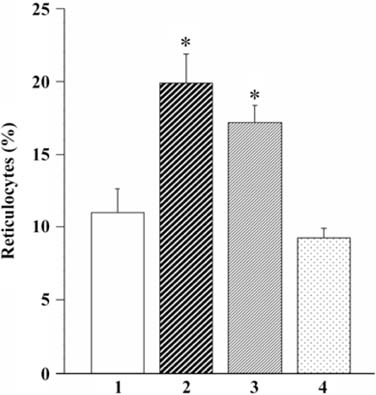
- The present study evaluated the effects of infected culture supernatant of erythrocytes, fractionation of culture supernatant and serum from dogs infected with Babesia gibsoni (B. gibsoni) on the maturation of canine reticulocytes in vitro. The SDS-PAGE demonstrated that significantly broader bands were generated by both the infected culture supernatant of erythrocytes and the serum from dogs chronically infected with B. gibsoni. The culture supernatant of erythrocytes infected with B. gibsoni strongly suppressed the maturation of reticulocytes. Prior studies showed that chronically infected serum had inhibitory effects on both the maturation of reticulocytes and the canine pyrimidine 5'-nucleotidase subclass I and purine-specific 5'-nucleotidase activity. In addition, serum free infected culture supernatant of erythrocytes had an inhibitory effect on the morphological maturation of reticulocytes. These results suggest that infected serum and culture supernatant of erythrocytes might accumulate excess proteins and/or metabolites as a result of the inhibited maturation of reticulocytes and decreased activity of erythrocyte 5'-nucleotidase. Furthermore, the fractions observed at >150 kDa- and 150-70 kDa- in the infected culture supernatant and serum retarded the maturation of canine reticulocytes in vitro. The results obtained from the in vitro examinations, in the present study, suggested that B. gibsoni itself and/or its metabolites might release certain proteins in the infected culture supernatant and serum from infected dogs and as a result delay morphological maturation of canine reticulocytes.
Keyword
MeSH Terms
Figure
Reference
-
1. Botros BAM, Moch RW, Barsoum IS. Some observations on experimentally induced infection of dogs with Babesia gibsoni. Am J Vet Res. 1975. 36:293–296.2. Carson CA, Phillips RS. Ristic M, Kreier JP, editors. Immunologic response of the vertebrate host to Babesia. Babesiosis. 1981. New York: Academic Press;411–443.3. Carcy B, Precigout E, Valentin A, Gorenflot A, Schrevel J. A 37-kilodalton glycoprotein of Babesia divergens is a major component of a protective fraction containing low-molecular-mass cultured-derived exoantigens. Infect Immun. 1995. 63:811–817.
Article4. Farwell GE, LeGrand EK, Cobb CC. Clinical observations on Babesia gibsoni and Babesia canis infections in dogs. J Am Vet Med Assoc. 1982. 180:507–511.5. Groves MG, Dennis GL. Babesia gibsoni: field and laboratory studies of canine infections. Exp Parasitol. 1972. 31:153–159.6. Hossain MA, Yamato O, Yamasaki M, Jeong JR, Chang HS, Maede Y. Serum from dogs infected with Babesia gibsoni inhibits maturation of reticulocytes and erythrocyte 5'-nucleotidase activity in vitro. J Vet Med Sci. 2003. 65:1281–1286.
Article7. Hossain MA, Yamato O, Yamasaki M, Maede Y. Inhibitory effect of pyrimidine and purine nucleotides on the multiplication of Babesia gibsoni: possible cause of low parasitemia and simultaneous reticulocytosis in canine babesiosis. J Vet Med Sci. 2004. 66:389–395.
Article8. Hossain MA, Yamato O, Yamasaki M, Otsuka Y, Maede Y. Relation between reticulocyte count and characteristics of erythrocyte 5'-nucleotidase in dogs, cats, cattle and humans. J Vet Med Sci. 2003. 65:193–197.
Article9. Jain NC. Hematological techniques. Schalm's Veterinary Hematology. 1986. 4th ed. Philadelphia: Lea & Febiger;20–86.10. Kawamura M, Maede Y, Namioka S. Mitogenic responsibilities of lymphocytes in canine babesiosis and the effects of splenectomy on it. Jpn J Vet Res. 1987. 35:1–10.11. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970. 227:680–685.
Article12. Maede Y, Inaba M. (Na,K)-ATPase and ouabain binding in reticulocytes from dogs with high K and low K erythrocytes and their changes during maturation. J Biol Chem. 1985. 260:3337–3343.
Article13. Mons B. Preferential invasion of malarial merozoites into young red blood cells. Blood Cells. 1990. 16:299–312.14. Murase T, Iwai M, Maede Y. Direct evidence for preferential multiplication of Babesia gibsoni in young erythrocytes. Parasitol Res. 1993. 79:269–271.
Article15. Murase T, Maede Y. Increased erythrophagocytic activity of macrophages in dogs with Babesia gibsoni infection. Nippon Juigaku Zasshi. 1990. 52:321–327.
Article16. Murase T, Ueda T, Yamato O, Tajima M, Maede Y. Oxidative damage and enhanced erythrophagocytosis in canine erythrocytes infected with Babesia gibsoni. J Vet Med Sci. 1996. 58:259–261.
Article17. Mya MM, Roy A, Saxena RK, Roy KB. Isolation, part characterization, immunogenicity, and specificity study of Plasmodium falciparum culture supernatant. Jpn J Infect Dis. 2002. 55:150–156.18. Otsuka Y, Yamasaki M, Yamato O, Maede Y. The effect of macrophages on the erythrocyte oxidative damage and the pathogenesis of anemia in Babesia gibsoni-infected dogs with low parasitemia. J Vet Med Sci. 2002. 64:221–226.
Article19. Otsuka Y, Yamasaki M, Yamato O, Maede Y. Increased generation of superoxide in erythrocytes infected with Babesia gibsoni. J Vet Med Sci. 2001. 63:1077–1081.
Article20. Ray K, Rao TS, Sivaraman CA, Basu RN. Studies on in vitro culture supernatant of P. falciparum. Isolation of antigens for serology. Ann Trop Med Parasitol. 1987. 81:101–103.
Article21. Valentine WN, Fink K, Paglia DE, Harris SR, Adams WS. Hereditary hemolytic anemia with human erythrocyte pyrimidine 5'-nucleotidase deficiency. J Clin Invest. 1974. 54:866–879.
Article22. Yamasaki M, Asano H, Otsuka Y, Yamato O, Tajima M, Maede Y. Use of canine red blood cell with high concentrations of potassium, reduced glutathione, and free amino acid as host cells for in vitro cultivation of Babesia gibsoni. Am J Vet Res. 2000. 61:1520–1524.
Article23. Yamasaki M, Otsuka Y, Yamato O, Tajima M, Maede Y. The cause of the predilection of Babesia gibsoni for reticulocytes. J Vet Med Sci. 2000. 62:737–741.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Molecules in Babesia gibsoni and Their Application for Diagnosis, Vaccine Development, and Drug Discovery
- Clindamycin-doxycycline-metronidazole combination therapy in a refractory canine babesiosis case
- Modeling of transmission pathways on canine heartworm dynamics
- Detection and molecular characterization of Hepatozoon canis, Babesia vogeli, Ehrlichia canis, and Anaplasma platys in dogs from Metro Manila, Philippines
- Studies on Timing of the Oocyte Maturation Progression in Maturation of the Mouse Oocyte in Vitro